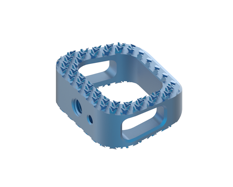
The device, which includes CTL Medical’s proprietary TiCro surface technology, has 200% greater endplate contact surface area, according to CTL. The Matisse also has bone-conforming geometry for better mechanical locking at the cage and bone interface.
The implant includes a tapered leading edge for easy insertion, and a large graft area is meant to better promote bony fusion. CTL Medical is offering the Matisse device in a variety of sizes and configurations for improved customization.
“Countless engineering hours and R&D resources went in to developing CTL Medical’s patent pending TiCro surface technology, which was designed to expand our current portfolio, advance fusion technologies, and offer spine surgeons numerous surgical advantages,” said Danny Chon, president and CEO of CTL Medical Corp., in a July 26 news release.
“The Matisse system includes streamlined instrumentation and a variety of footprints, heights and lordotic profiles to accommodate variations in patient anatomy,” Chon said.
FDA has indicated the Matisse titanium ACIF cage with TiCro for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one-disc level.
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]