
Heartpoint’s IntelliStent system is designed to use minimally invasive catheter delivery to place adjustable stents that adjust blood flow in the pulmonary arteries. [Illustration courtesy of Heartpoint]
The Coral Gables, Florida–based startup recently won the Transcatheter Cardiovascular Therapeutics (TCT) conference’s Shark Tank Innovation Competition and is now preparing to submit its technology to the FDA for breakthrough device designation.
Heartpoint Global designed its multi-lumen IntelliStent system to restrict the blood flow in the arteries between the heart and lungs. The system can also allow a physician to later adjust the patient’s blood flow as they grow or their condition changes.
The system includes self-expanding nitinol stents that can be placed and adjusted in the main pulmonary artery or its branches with minimally invasive catheter procedures instead of a sternotomy. The technology could prevent or act as a bridge to heart transplants, artificial hearts and other heart surgeries.
“HeartPoint Global’s pioneering approach to treating pulmonary arterial hypertension caused by congenital heart disease and pulmonary arterial hypertension (PAH-CHD) stood out due to its potential to address a significant unmet clinical need,” Cardiovascular Research Foundation CEO and President Dr. Juan Granada said in an announcement of the company’s win at TCT.
“We’re focused first on pulmonary arterial hypertension caused by congenital heart disease,” Heartpoint Global CEO and Chair Seth Bogner said in an interview with Medical Design & Outsourcing. “… This multi-lumen method of adjustability is already patented, and it’s applicable in every blood-carrying vessel in the body.”
How Heartpoint designed IntelliStent
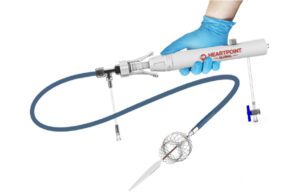
Heartpoint plans to submit its IntelliStent system for regulatory approval in 2025. [Image courtesy of Heartpoint]
The catheter system deploys the self-expanding stent in the main pulmonary artery and/or the right or left pulmonary arteries to restrict blood flow. To further restrict blood flow, another stent can be placed the same way inside the first stent.
If more blood flow is needed later, the stents can be removed with a procedure similar to the minimally invasive catheter placement. Bogner said Heartpoint is patenting a proprietary balloon stent that could also be used to increase flow as necessary.
The nitinol stents are partially coated with a nonpermeable polyurethane polymer and have radiopaque markers for placement visualization.
The nitinol implants expand as they exit the catheter delivery system thanks to the nickel-titanium alloy’s superelastic properties, and they maintain their shape at body temperature due to nitinol’s shape memory properties, Bogner said.
In animal studies so far, the implants reduced pulmonary pressure and endothelialized quickly but have not caused thrombosis or damaged the pulmonary artery or heart valves, Bogner said.
What’s next for Heartpoint and its IntelliStent system

Heartpoint Global’s IntelliStent system includes a catheter delivery system, nitinol reduction stents and a balloon expandable stent. [Image courtesy of Heartpoint]
Bogner said he also sees potential for additional cardiac indications such as other kinds of pulmonary hypertension, congenitally corrected transposition of the great arteries (CCTGA), or to take the guesswork out of occluder sizing. The technology might also one day be used to help treat liver tissue interstitial fluid (TIF) in cirrhosis patients.
“There are a lot of licensing opportunities and engineering opportunities because there are existing devices that could use our technology,” he said. “… A lot of engineers are going to be happy for a very long time.”

Heartpoint Global CEO and Chair Seth Bogner [Photo courtesy of Heartpoint]
“We’re an American company that has European and Israeli subsidiaries, and we’re very focused on getting this into Europe as quickly as possible,” Bogner said.




