Jubilant DraxImage Inc., a wholly owned subsidiary of Jubilant Pharma Ltd, announced that the U.S. Food and Drug Administration has approved Ruby-Fill, an innovative technology for Positron Emission Tomography (PET) myocardial perfusion imaging (MPI).
Comprised of a Rubidium-82 (Rb-82) generator and precedent setting elution system, Ruby-Fill is used to produce a personalized patient dose of Rubidium Rb 82 chloride used to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease (CAD), which is an important component of diagnosing CAD.
“We are proud to bring to the U.S. market a groundbreaking, state-of-the-art technology for myocardial perfusion imaging. Ruby-Fill expands DraxImage’s nuclear medicine portfolio and is a part of our commitment to provide healthcare providers and their patients with innovative health care solutions for those with suspected or existing coronary artery disease,” comments GP Singh, CEO of Jubilant Pharma Ltd.
“Our knowledge of the role and value of PET nuclear cardiology, specifically Rb-82 Chloride PET in known or suspected coronary artery disease, advanced significantly as we progressed through the comprehensive and rigorous FDA review process,” said Norman LaFrance, M.D., chief medical officer and senior VP, medical & regulatory affairs for Jubilant Pharma and Jubilant DraxImage. “With its advanced weight based dose accuracy and multiple infusion options, among other product capabilities, Ruby-Fill will enhance the way patients with known or suspected coronary artery disease are both diagnosed and managed.”
Commercial launch plans for Ruby-Fill is expected to be in the October-December 2016 quarter.
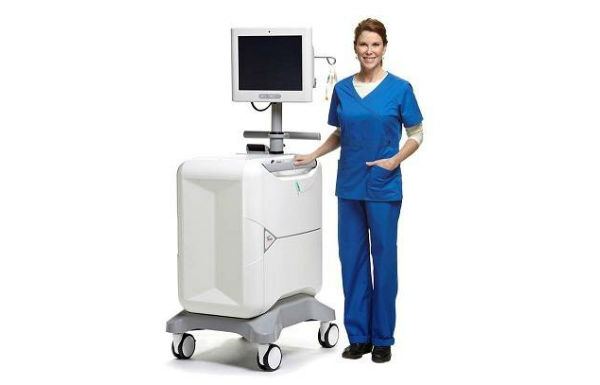
The Rubidium elution system has been exclusively designed to be used with the Ruby-Fill generator and to deliver accurate doses of Rb-82 Chloride to patients. (Credit: Jubilant DraxImage Inc. at http://www.draximage.com/en/pipeline/cardiovascular-pet.html)
Ruby-Fill Rubidium 82 Generator and Ruby Rubidium Elution System
The Ruby-Fill generator contains an accelerator produced Strontium-82, which decays to Rubidium-82. When the generator is eluted with saline it produces a sterile, non-pyrogenic solution of Rb-82 Chloride.
Due to the short half-life (75 s) of Rb-82, the use of an elution system is required for delivery of the Rb-82 Chloride into a patient for the purposes of performing Myocardial Perfusion Imaging with PET. PET imaging with Rb-82 Chloride may be performed under rest and/or stress conditions.
The Rubidium Elution System has been exclusively designed to be used with the Ruby-Fill generator and to deliver accurate doses of Rb-82 Chloride to patients.
For more information: Medical and regulatory affairs medicalaffairs@jdi.jubl.com; http://www.draximage.com/en/pipeline/cardiovascular-pet.html.
About Jubilant DraxImage
Jubilant DraxImage Inc., a wholly owned subsidiary of Jubilant Pharma Ltd, which is held by Jubilant Life Sciences Ltd, develops, manufactures and commercializes radiopharmaceuticals used for the diagnosis and treatment of diseases. The company is dedicated to nuclear medicine and serves customers and through them patients, globally, with high quality and reliable products and services. www.draximage.com.
(Source: Business Wire)




