The pacemaker has certainly had an interesting journey from its inception—it started as a hand-cranked box that, ironically, scared the life out of people because it leaned a little too close to Frankenstein-esque reanimation. Since then, the smallest model (Medtronic’s Micra TPS) is about the size of a large vitamin, with a battery life of up to ten years.
Medtronic is definitely on the cutting edge of pacemaker technology now, but back in the early 1970’s the medtech giant teamed up with the French company Alcatel to design a pacemaker with a truly innovative power source: plutonium. That’s right—at one point, pacemakers were nuclear powered.
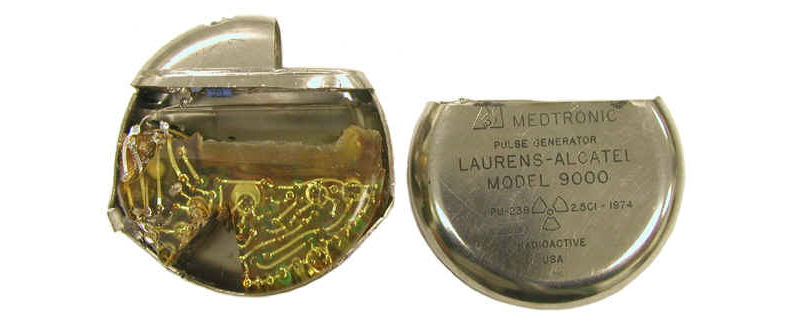
Medtronics “Laurens-Alcatel Model 9000” pacemaker, pictured with plutonium removed. (Credit: Oak Ridge Associated Universities)
Medtronic’s nuclear battery contained a small 2.5 Ci slug of Plutonium 238 (Pu-238). Pu-238’s radiation struck the walls of its container, generating heat that a thermophile (a stack of thermocouples) converted into the electric current that simulates the heart.
The company wasn’t alone in manufacturing pacemakers in this way: in the U.S., the Cordis Corporation and Coratomic Inc. also made nuclear pacemakers. As of 2003, it was estimated that between 50 and 100 people still wear nuclear-powered pacemakers.
Dose rates at the pacemaker’s surface are about 5 to 15 mrem per hour. Whole body exposure for the wearer is about 0.1 rem per year for the patient—and 7.5 mrem per year to the spouse of a patient.
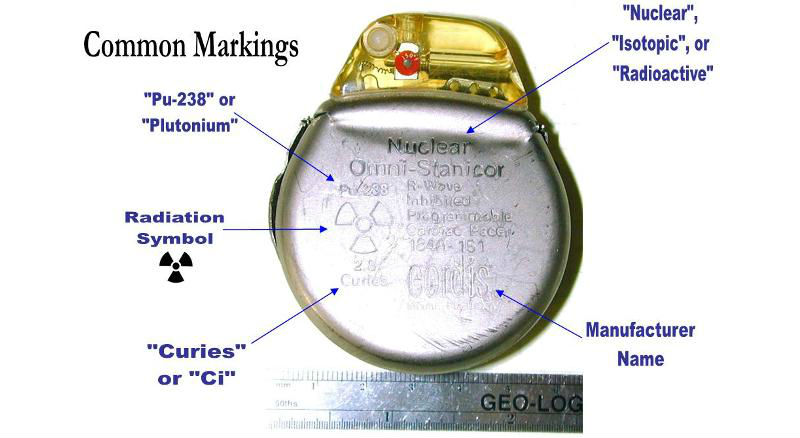
Cordis Corporation’s nuclear pacemaker (Credit: OSRP)
One of those people is a woman who had a nuclear pacemaker—the Numec NU-5—implanted in 1973, says the device was still functioning adequately 34 years later, in 2007. A normal pacemaker would’ve required replacement four or five times in that time. In fact, according to Dr. Victor Parsonnet in an interview with Reuters, even after 88 years, when half the plutonium would’ve decayed, the batteries would still have enough power. For comparison, lithium pacemakers last 10 to 15 years.
Due to the extremely long battery life, nuclear pacemakers tended to outlive their users, and couldn’t be buried with them due to radiation exposure risks. Should you encounter a radioactive pacemaker (whether within yourself or a family member or friend) contact Los Alamos’ Laboratory’s Off-Site Recovery Project (OSRP).
If you have a compelling story about medtech history, please contact me at sam.brusco@advantagemedia.com. I’d love to hear about it, and I’m sure our readers would too!




