Dr. Michael Jaff is chief medical officer and VP of the Boston Scientific Peripheral Interventions business. [Photo courtesy of Boston Scientific]
“Every really great technological advancement in the vascular space has come from a physician’s recognition that they would like to be able to do something safely and effectively for their patients, but the technology does not exist,” Jaff said in an interview with Medical Design & Outsourcing. “They find their way to an engineer, someone really smart takes that concept builds a prototype — off to the races. And it has time and time again transformed the way patients can be cared for.”
RWE leverages modern data collection and analysis to help device developers understand limitations and opportunities from frontline data spanning many clinicians and health systems.
“Now you see how multiple physicians are taking care of patients with conditions, seeing how they’re working, how they’re not working, where the limitations are. And that automatically sheds a light on opportunity for either iterative change or full-scale change in a management strategy,” Jaff said. “I am — as a vascular doctor — very excited about the impact real-world evidence can have on developing new therapies.”
Related: 5 keys to Mike Mahoney and Boston Scientific’s success
RWE has come a long way in recent years. Because it’s based on health record coding, those records have been limited in how much they can tell device developers beyond general categories such as chronic obstructive lung disease, congestive heart failure and critical limb ischemia.
“What we now have the opportunity to do is actually scour multiple electronic health record databases. Every piece of line item information that is entered into an electronic health record gets put into a central data repository that then can be queried directly,” Jaff said.
Those electronic health records offer more detail — for example, on what happens to a patient’s kidney function after a procedure that uses IV contrast, or information about a complication resulting from a procedure using a particular technology.
“That’s hard to get when you look at administrative databases. You can’t really match specific dates to events. Now I can tell dates and hours that something happens,” Jaff said. “So you really can link the adverse event that could be modified by a better technology by being able to scour that type of information. I think that’s the game-changer.”
He advised device developers getting started with RWE to set realistic goals from the beginning by picking a straightforward project and getting comfortable with a third-party RWE platform. (Boston Scientific works with Truveta.)
It takes some training, but Jaff said he’s had team members without formal data science backgrounds get good at querying RWE with practice.
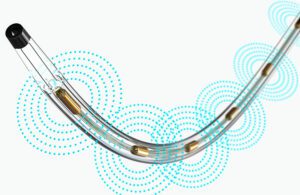
Real-world evidence can help device developers innovate with new or improved designs. Pictured is Boston Scientific’s EkoSonic Endovascular System (EKOS), which uses ultrasound energy combined with a thrombolytic drug to treat blood clots. [Image courtesy of Boston Scientific]
“We learned in this Truveta database there was a higher prevalence of younger patients with PAD than we would have imagined,” Jaff said. “That was an eye-opener to us. Then we started looking at things like gender, race, socioeconomic class, social determinants of health — these are all things that are in electronic health records. The ability to take that information and do the work that we’re doing on healthcare disparities has really been phenomenal.”
New patient insights could inform device design by exposing gaps in clinical trials. Women, for example, aren’t as commonly studied in clinical trials, so discovering a certain presentation pattern in women could lead to changes that improve patient outcomes. One example Jaff offered was if more women were found to present with disease in the distal end of the popliteal artery.
“You can’t just assume that because [your technology] works in the global population of patients with PAD that it’s always going to work in those patients,” he said. “Maybe we need a smaller diameter device, maybe we need a longer device, maybe we need to figure out a way to screen for these patients earlier to identify before they present with more advanced disease. So the the implications can be quite vast.”
He also offered advice for device designers trying to forge a path ahead in device innovation to consider the timeliness of RWE versus the long-standing ideal of randomized clinical trials.
“By the time a randomized trial is completed and published, the technology is already several years old, because those trials take a long time. They’re on a very selected patient population — not the population that’s routinely managed by physicians, but a subgroup of those patients,” Jaff said. “So as people who are developing new therapies are thinking about, ‘What’s my next strike opportunity? Where can I really go?’ I would urge them to think about looking into real-world evidence to help guide them.”