Silora R&D – safely connecting medical and non-medical equipment with patient safety (Credit: PR Newswire)
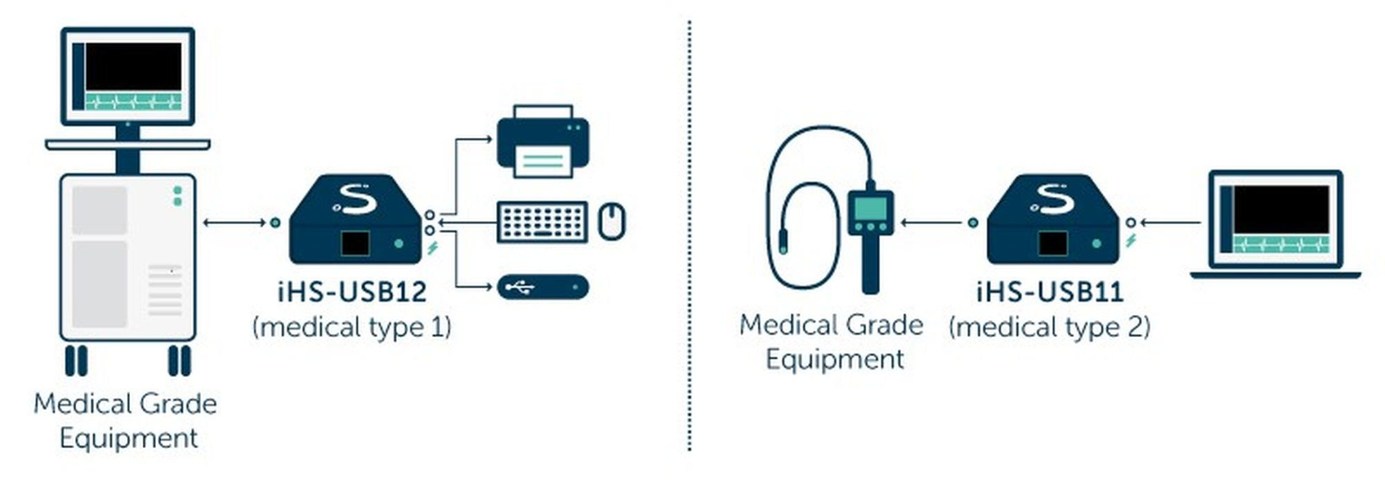
SILORA R&D, a multimedia and switching solutions provider for medical equipment manufacturers, announced the launch of two medical grade USB isolators: iHS-USB12t1 and iHS-USB11t2 for advanced isolation of medical equipment from non-medical grade accessories and peripherals.
The isolators support all USB 2.0 device types at up to 480Mbps and work with all Operating Systems.
The new iHS-USB11t2 is a USB 2.0 high-speed medical grade isolator that holds double isolation of up to 5KV for both power and data achieving ‘type 2’ certification. The isolator provides one upstream facing port with a High retention B-type USB 2.0 connector and one isolated downstream facing port with a standard A-type USB2.0 connector.
The iHS-USB11t1 is a USB 2.0 High-speed medical grade isolated USB2.0 HUB with a single isolation of up to 3KV for both power and data achieving ‘type 1’ certification. The isolator provides one isolated upstream facing port with a High retention B-type USB 2.0 connector and two downstream facing ports with standard A-type USB2.0 connectors.
Each isolator shows off an impressive level of certification with acquired safety in EN 60601-1
(3.1th edition) and EMC EN 60601-1-2 (4th edition).
“We are glad to help healthcare facilities lower costs of infrastructure and equipment by using standard off-the-shelf products and obtaining the required medical grade certification using our isolators”, says Eli Shubi, CEO at SILORA R&D.
A demonstration and additional information about the new technology will be showcased at MEDinIsrael March 6th -9th, Tel Aviv at the SILORA R&D Booth #D61.


