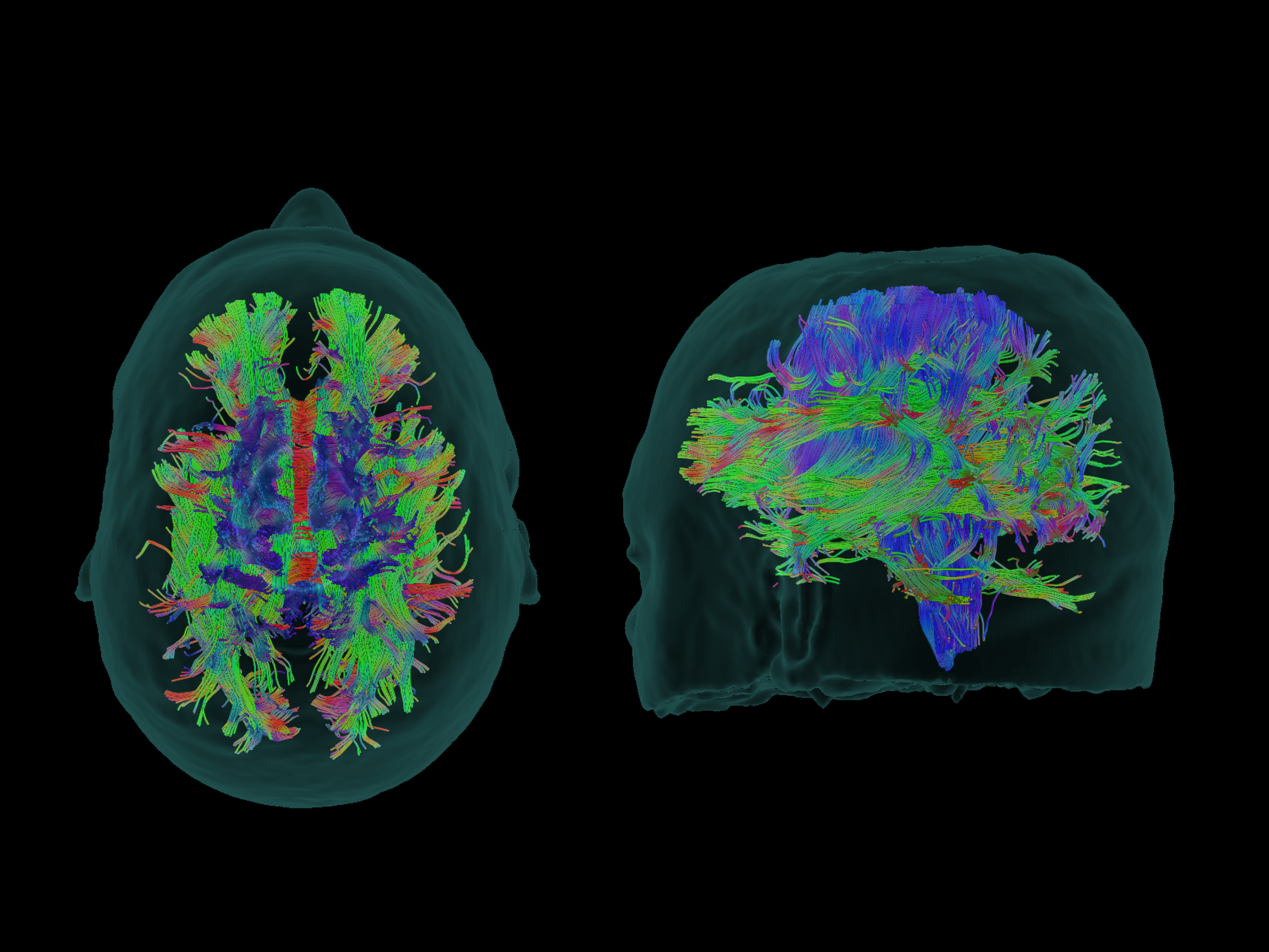
BrightMatter Plan by Synaptive Medical automatically generates 3D, dynamic whole brain tractography using an advanced type of MRI called diffusion tensor imaging to provide a complete picture, representing the white matter in the brain. The software makes this unprecedented automation and level of detail clinically available for the first time. (Credit: Business Wire)
Synaptive Medical Inc., a medical device and technology company, has received its first CE Mark as well as regulatory approval in Australia. The approval opens European and Australian markets to BrightMatter Plan, a neurosurgical planning system that automatically generates dynamic tractography, enabling surgeons to easily create preoperative plans that allow for less disruptive, more accurate surgery.
“Europe and Australia are home to many highly influential clinician-researchers in neurosurgery. We look forward to opportunities to collaborate and understand their pre-operative planning needs,” says Cameron Piron, Synaptive’s President and co-founder. “We anticipate that Plan will be the first of many Synaptive products to receive regulatory approval to support better outcomes for patients in Europe and Australia.”
BrightMatter Plan is a part of Synaptive’s innovative solution that includes advanced imaging, informatics, surgical planning, navigation and robotic visualization with a digital microscope for brain surgery. BrightMatter Plan by Synaptive Medical automatically generates 3D, dynamic whole brain tractography using an advanced type of MRI called diffusion tensor imaging to provide a complete picture, representing the white matter in the brain. The software makes this unprecedented automation and level of detail clinically available for the first time. With this information, BrightMatter Plan allows surgeons to easily explore multiple approaches to determine the least invasive access point and safest route to the surgical target.
This enhanced pre-surgical visualization may contribute to reduced complications, and may enable access to previously inaccessible brain locations. The resulting tractography and surgical plan can then be exported to navigation systems, such as BrightMatter Guide, for use in the operating room.
“This is a key year for our international expansion as Synaptive scales up in global healthcare markets,” says Piron. “Expanding our presence in the UK, Ireland, Germany, Switzerland, the Nordic countries, Singapore, and Australia is another step toward bringing Canadian innovation to the world.”
BrightMatter Plan is FDA, CE Mark, Health Canada and TGA approved.




