
Synchron Chief Commercial Officer Kurt Haggstrom [Photo courtesy of Synchron]
The device developers each have FDA investigational device exemption (IDE) for their experimental BCIs. They’ve also got billionaires backing their R&D and regulatory efforts, with Neuralink owned by Elon Musk and Synchron funded by Jeff Bezos and Bill Gates.
This month, Synchron announced the completion of patient enrollment in its COMMAND clinical trial. Two weeks later, Neuralink announced the start of recruiting for its own clinical trial, the PRIME first-in-human study.
With BCI technology advancing as one of the hottest spaces in medtech innovation, Synchron Chief Commercial Officer Kurt Haggstrom discussed the competitive landscape and race for FDA approval in a recent interview with DeviceTalks Editorial Director Tom Salemi.
“The race in my mind is I’m not necessarily racing the competition,” he said. “I’m racing to help out the patient because we work with them every single week. We are in their homes working with them, and the ones that we don’t work with, we’re talking to because we want to understand their needs and what that tool and technology ultimately needs to look like.”
DeviceTalks West: Haggstrom will discuss BCIs and Synchron’s technology in Santa Clara, California on Oct. 18
With so much potential for BCI technology, a critical challenge will be remaining selective about how to apply the startup’s limited resources, prioritizing patient needs to develop a system that addresses core patient needs.
“It is all about focus. … In this space, because of the novelty and how the brain works and all the signals that you can read, you can easily get distracted doing different activities [like] controlling robotic arms and all these things,” Haagstrom said. “But ultimately, what they need is a tool they can use every day to be able to reconnect to the world. So for me, the race is keeping our team focused on our spend.”
He said he wants healthy competition because more BCI companies, research and attention on the space will yield more innovation for patients.
“I certainly welcome the competition, and I think it’s all good,” he said. “But to me, we keep ourselves held to that race and focus to get a product out there to help people as soon as possible.”
Synchron’s path for regulatory approval and commercialization
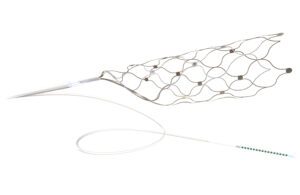
The Synchron Stentrode brain implant expands inside a blood vessel, placing its electrodes against the vessel wall to sense brain signals. [Image courtesy of Synchron]
Previously: Catheter delivery could enable better brain implants: Synchron’s neuroscience chief explains how
“There’s no quick path to get to market with an innovation like this,” Haagstrom said. “It has to go through the traditional Class III medical device route we all know from an implantable standpoint … all the same safety regulations, performance regulations. We’re going to take that path and partner with the FDA to take this to market.”
As of the time of the interview, Synchron was still working with the FDA on the proposed indication and wrapping up the COMMAND early feasibility study evaluating the safety and efficacy of Synchron’s Stentrode implant in paralyzed patients.
Next up is what Synchon hopes will be a pivotal study for PMA approval, and then it’s on to commercialization.
“When you look at commercializing an innovation like this, for me, simplicity is key. … Out of the box, calibrating these things, it has to be really usable for patients. And that’s really where we’re centered around,” he said.
Listen to the whole conversation at DeviceTalks or below.




