(Credit: Wyss Institute)
Every device implanted in the body or in contact with flowing blood faces two critical challenges that can threaten the life of the patient it is meant to help: blood clotting and bacterial infection.
To confront this challenge, Wyss Institute researchers created a super-repellent, Thin Layer Perfluorocarbon (TLP) coating specifically designed to prevent clot formation and biofilm formation when adhered to existing medical devices.
The coating consists of a chemically inert perfluorocarbon material that is already approved by the Food and Drug Administration (FDA) for applications such as liquid ventilation, blood substitution, eye surgery, and more.
Inspired by the Wyss’s Slippery Liquid-Infused Porous Surfaces (SLIPS) coating technology that creates omniphobic slippery surfaces by infiltrating porous or roughened surfaces with liquid, another Wyss team developed a two-step process that can render existing medical-grade smooth surfaces broadly repellent.
First a thin layer of perfluorocarbon is chemically bound and tethered to the surface of materials ranging from plastic to glass and metal, and then is coated with a mobile thin layer of liquid perfluorocarbon, that already has been used in FDA-approved medical devices, to create a super-repellent TLP coating.
When applied to catheters and perfusion tubing, TLP repels whole blood including dedicated blood components that induce clotting reactions as well as bacteria that lead to the formation of infectious biofilms.
In studies in pigs, the TLP coating made it possible to implant an arteriovenous (AV) shunt within large vessels in direct contact with blood for eight hours without producing occlusion even in the absence the commonly administered anti-coagulant heparin, which is known to significantly elevate the risk of accidental bleeding in patients.
The TLP coating significantly reduced the risk of Central-Lines Mediated Bloodstream Infections as well.
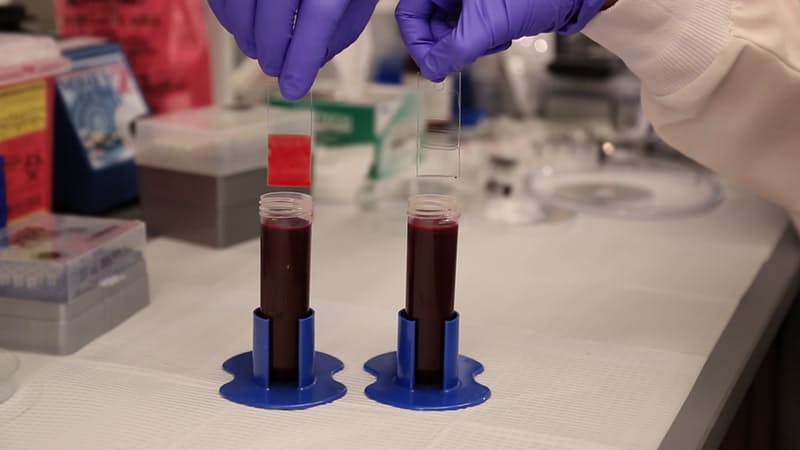
These glass slides were dipped in blood to demonstrate the effectiveness of the TLP coating. While blood sticks to the untreated slide on the left, the TLP-treated slide on the right emerges entirely clear. (Credit: Wyss Institute at Harvard University)
Adding to their clinical applicability, TLP surfaces remain stable under clinically relevant shear stresses, or rates of blood flow seen in catheters, central lines and dialysis machines. TLP-treated tubing also can be stably stored for more than a year under normal temperature and humidity conditions.
TLP has been licensed.
*The banner image is a Scanning Electron Microscope (SEM) image showing how red blood cells coagulate to form a blood clot, which is a common and life-threatening risk associated with the use of implanted medical devices. Credit: Wyss Institute at Harvard University.


