The 2018 Excellence in Surgical Products Awards will be presented in the November/December issue of Surgical Products. Leading up to the publication of that issue, nominees will be featured on the Surgical Products website.
Category: Safety
Product description and innovation synopsis:
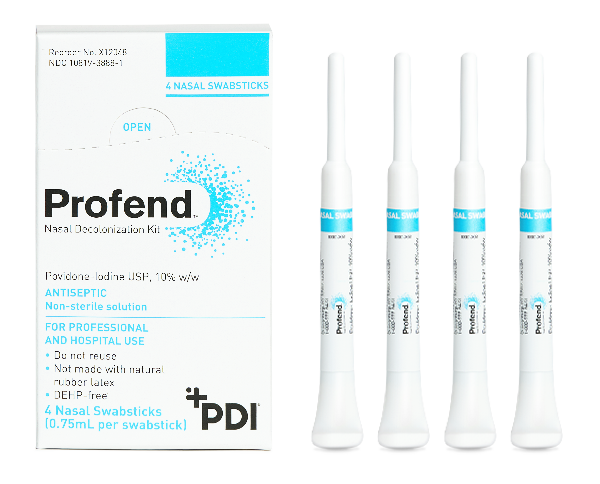
The quick, easy, assured protection of the Profend Nasal Decolonization Kit allows healthcare personnel to proactively defend their patients and facilities.
Profend offers:
- Uniquely designed swabsticks pre-saturated with 10 percent Povidone-Iodine (PVP-I)—just snap and swab.
- Fast 60-second treatment time.
- Neat, dry-handle design for limited mess.
- Small nasal swab for patient comfort.
- Kills 99.7 percent of S. aureus at 1 hour and 99.9 percent at 12 hours.
(Image credit: PDI)
What sets this product apart from others available in the industry?
The Profend Nasal Decolonization Kit makes nasal decolonization easy. The uniquely designed swabsticks are pre-saturated with 10 percent Povidone-Iodine (PVP-I), allowing for clinicians to just snap and swab. The total treatment time is 60 seconds, up to 2.5x faster than other PVP-Iodine products. Unlike Mupirocin, the PVP-I formulation has no evidence of S. aureus/MRSA resistance, supporting antimicrobial stewardship goals.
How does this product benefit the surgical team and aid in better outcomes?
In addition to the above, the Profend Nasal Decolonization Kit is preferred by more than 90 percent of clinicians over other Povidone-Iodine (PVP-I) nasal antiseptic products, and 100 percent of clinicians felt patients would prefer Profend.




