Contego Medical recently announced that the FDA has granted 510(k) clearance for the first filter-based Integrated Embolic Protection (IEP) device, the Paladin Carotid PTA Balloon System. Contego Medical is developing and commercializing a suite of next-generation devices that address unmet needs in neurovascular, coronary, and peripheral vascular disease.
The Paladin Carotid PTA Balloon System with Integrated Embolic Protection contains an angioplasty balloon and integrated 40-micron filter, coupled together for the first time. The micro-embolic protection provided by the Paladin filter, designed for maximum capture efficiency, provides an added measure of protection and procedural flexibility not seen before in carotid stent procedures. It is the first system which allows the physician to adjust the size of the embolic protection filter in vivo to suit each individual patient’s anatomy.
The Paladin System has CE Mark and has been used in over 1,400 patients in Europe with excellent results. It has been studied in 106 registry patients undergoing carotid artery stenting with a variety of stents, including open cell, closed cell, and mesh covered designs, with zero procedural strokes and a 30-day risk of death, stroke, and myocardial infarction of 0.9 percent.
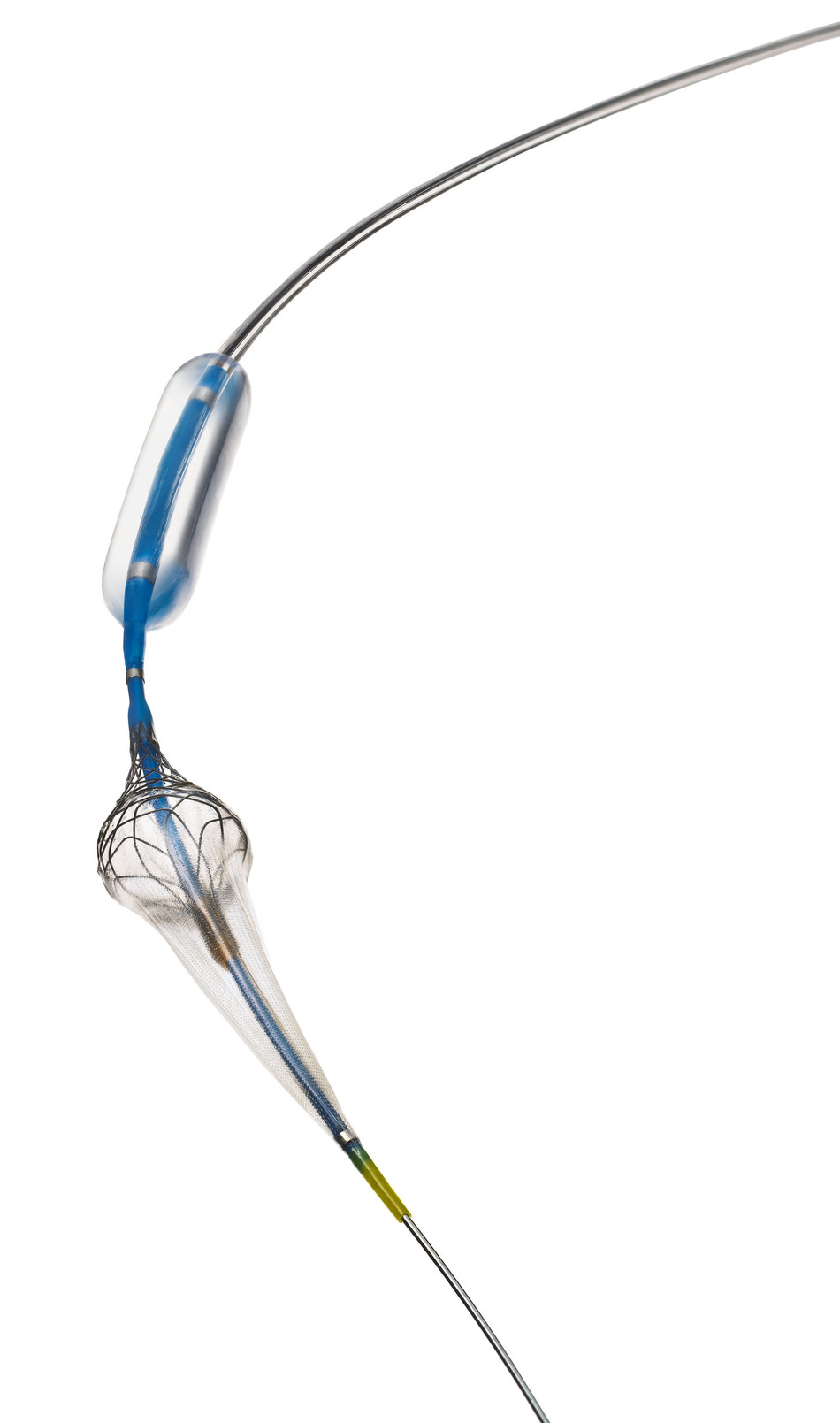
(Image credit: Contego Medical)
“Minor stroke continues to be the Achilles heel of carotid artery stenting, which occurs as a result of micro-embolization during the procedure,” says William Gray, MD, system chief of the division of cardiovascular disease at Main Line Health in Wynnewood, Pennsylvania. “The Paladin System offers optimized micro-embolic protection during the most vulnerable aspects of the procedure, without additional device transitions or procedural time.”
“We are thrilled to have achieved this milestone for the first of several devices Contego Medical intends to bring to the U.S. market,” says Ravish Sachar MD, founder and CEO of Contego Medical. “The Paladin System utilizes our patented Integrated Embolic Protection (IEP) technology, aimed at providing superior performance while improving the safety profile of existing cardiovascular procedures. With the strength of our clinical data and the unique promise of our IEP technology, we look forward to positively impacting patient care and outcomes.”
The Paladin System with Integrated Embolic Protection (IEP), is indicated for Percutaneous Transluminal Angioplasty (PTA) in the carotid arteries with capture and removal of embolic material. This device is also indicated for post-dilation of self-expanding stents in the carotid arteries with capture and removal of embolic material. The diameter of the arterial site for filter deployment should be no more than 7.0 mm. The Paladin System with IEP should always be used in conjunction with an available embolic protection device.




