DuPuy Synthes Spine took the occasion of the annual meeting of the North American Spine Society, taking place this week in Boston, to launch two new offerings in their line.
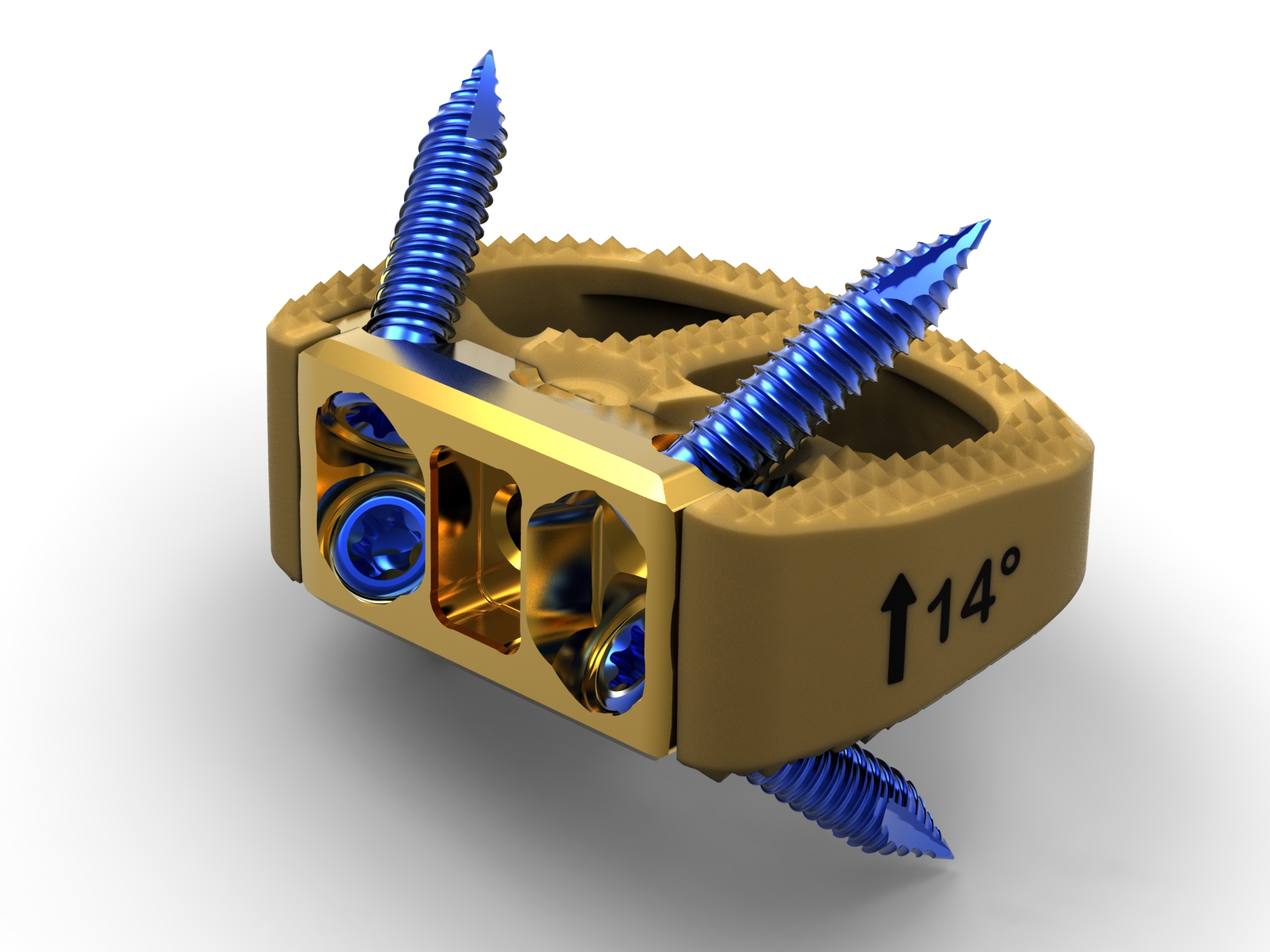
The SYNFIX Evolution System is designed for stand-alone Anterior Lumbar Interbody Fusion (ALIF) surgery. According to DuPuy Synthes promotional material, the implant “delivers biomechanical stability to promote fusion and restore function, coupled with instrumentation designed to optimize surgical workflow.”
(Image credit: DePuy Synthes Spine)
The manufacturer further notes that research around the SYNFIX indicates its biomechanical stability exceeds that of competing stand-alone ALIF implants on the market and is roughly equivalent to that of lumbar fusion performed through the back.
The implant includes a PEEK spacer, a titanium zero-profile plate, and a quartet of divergent locking screws. A thread lock sleeve crafted by the company’s design engineers for this product is intended to prevent the screwdriver from becoming disengaged in the midst of a procedure.
Additionally, DuPuy Synthes Spine introduced the ZERO-P NATURAL Plate. It is meant for spinal fusion procedures in the neck.

(Image credit: DePuy Synthes Spine)
The plate works in tandem with the CC Natural spacer. The components are assembled outside of the surgical field and then put into place together as part of an anterior cervical discectomy fusion (ACDF) procedure. Four locking screws keep it solidly it in place once it is implanted.
In an accompanying press release, Peyman Pakzaban, MD, FAANS, of Houston MicroNeurosurgery, touts the benefits of the device’s design.
“Compared to ACDF with a traditional plate and allograft, there is no need to expose the vertebral bodies beyond their endplates, resect anterior osteophytes, or remodel the anterior surface of the vertebral bodies,” Pakzaban says. “The insertion of a pre-fabricated allograft and the zero-profile plate is performed in one combined step.”




