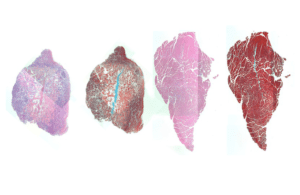
Stains for cellular components (pink) and muscle fibers (red) in mouse muscle injected with IL-4 nanoparticles show that a muscle that received treatment (right) displayed better regeneration of muscle cells and less empty space in the muscle tissue (white) than a muscle that did not receive treatment (left). [Image from Wyss Institute at Harvard University]
The technique involves attaching copies of IL-4 to nanoparticles of gold and then injecting them into injured muscles in mice to improve muscle structure and strength within two weeks of an injury.
“By exploiting the inflammatory response, this technology may significantly improve the therapeutic and functional outcomes of existing treatments that focus on the direct regeneration of muscle,” Theresa Raimondo, a graduate student in the Wyss Institute at Harvard University and first author on the study, said in a press release.
The system relies on macrophages, which are a type of immune cell that occurs in response to injury or infection. Macrophages are in a pro-inflammatory state, known as M1, when they find an injury or infection in the body. The M1 state promotes the release of inflammatory cytokines, antimicrobial peptides and other molecules that start the immune system response and promote new muscle cell growth. After that process, the macrophages move to the M2 state, which reduced inflammation and promoted muscle fiber maturing.
If there is an imbalance of M1- and M2-state macrophages or there are too many M1 macrophages at an injury site, muscle repair cannot happen. IL-4 can help macrophages switch from M1 to M2 and promote faster muscle fiber healing. The researchers note that getting Il-4 to gather at the target site has been a challenge in the past.
To address he challenge, the Harvard researchers tested Il-4 nanoparticles against free-floating IL-4 in living human cells. They found that nanoparticle-bound IL-4 was able to raining its biological function and resulted in a larger number of M2 macrophages than the unbound IL-4.
The researchers then tested the system by injecting IL-4 nanoparticles into the legs of mice that had injured shin muscles. The nanoparticles were injected within three days of injury and helped keep IL-4 in the injured muscle instead of moving to the bloodstream or neighboring tissue. Mice that received the treatment had a significant increase in muscl-efiber area after 15 days compared to mice that were treated with nanoparticles that didn’t have IL-4. Muscles that were treated with IL-4 nanoparticles could also contract with more force and speed than muscles that were injected with free floating IL-4.
IL-4 nanoparticle injection also doubled the percentage of M2 macrophages and reduced the number of M1 macrophages, according to the researchers. It also reduced the number of M1 macrophages compared to the muscles that did not receive IL-4 nanoparticles. Muscles that had free IL-4 injected had reduced M1 macrophages but did not have increased M2 macrophages. The researchers suggest that the joining of IL-4 to gold nanoparticles can improve the shift to an anti-inflammatory state to promote muscle regeneration.
“This work demonstrates that modulating the inflammatory response is a potent method for promoting the regeneration of functional tissues, and that IL-4 nanoparticles can promote the M2 macrophages phenotype in the context of injury in vivo, which opens the door to many exciting research directions,” David Mooney, a researcher on the study, said.
The researchers are now looking for ways to treat Duchenne Muscular Dystrophy in mice using IL-4 nanoparticles.
“These experiments will, hopefully, demonstrate that IL-4 nanoparticles can shift macrophage phenotype in the context of chronic inflammation, in addition to the acute injury studied in this work,” Raimondo said.
The researchers also plan to see if there is a direct interaction between IL-4nanoparticles and muscle-generating cells, as well as the role that macrophage phenotype in muscle regeneration plays in the context of muscular dystrophy.
“At the Wyss Institute, we always look for a better way of doing things – ‘good enough’ is not acceptable,” said Donald Ingber, founding director of the Wyss Institute. “Not only does this work represent a better method of delivering IL-4 to inflamed tissues, it also offers the possibility of a more effective treatment for chronic inflammatory diseases, which could improve many lives in the future.”
The research was published in the journal PNAS and was supported by the National Institutes of Health, the Wyss Institute at Harvard University and the NSF Graduate Research Fellowship Program.




