Houston-based manufacturer ReliantHeart, Inc., specialists in advanced mechanical circuitry assist technologies, recently announced they’ve earned CE Mark approval for the aVAD. The new left ventricular assist device (LVAD) is expected to see its first commercial usage in September.
In promotional materials, ReliantHeart attention to the aVAD’s small size (2.48 centimeters) outside of the ventricle and adjustable pump depth inside the ventricle. It further notes that “deep linear channels transport blood safely through the pump away from the dangerous forces of radial shear.” The power management approach used in the device means that it operates at “30% of the power consumption of other VADs.” Given that, the batteries on the device are said to be both smaller and longer-lasting.
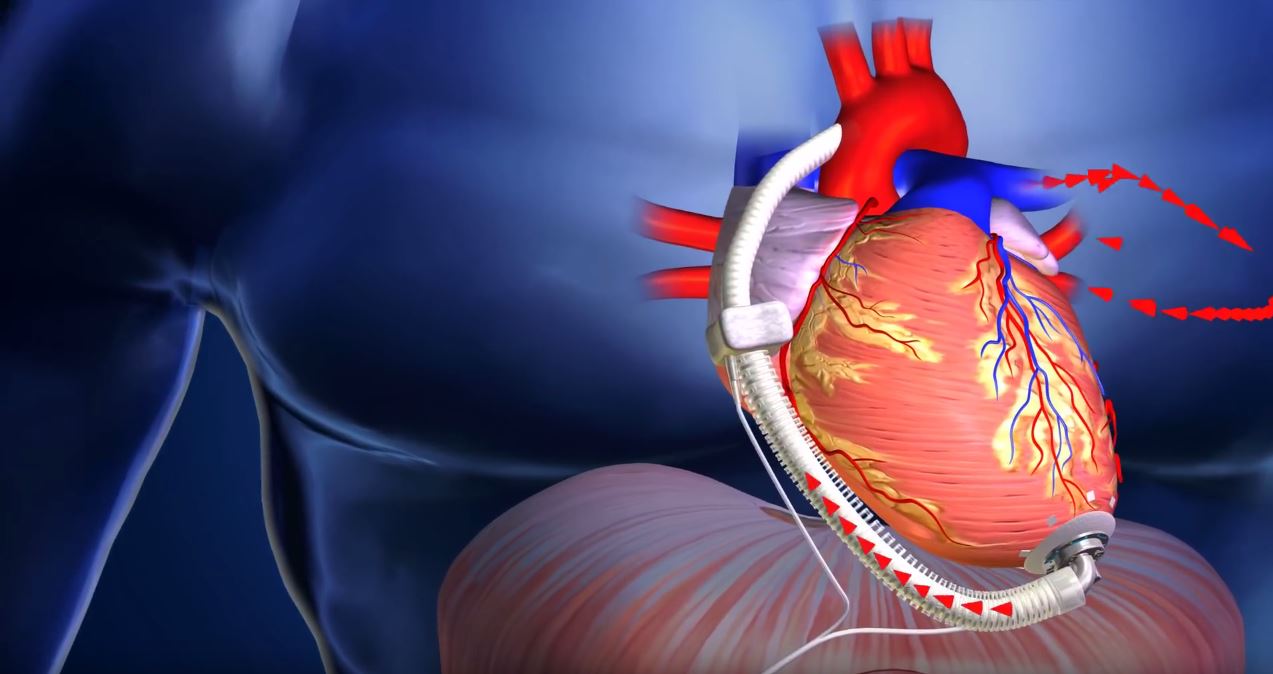
(Image credit: screengrab of ReliantHealth promotional video)
ReliantHealth also calls attention to the disconnectable driveline, allowing for easy treatment and replacement in the event of driveline infection, and constant remote monitoring, with early warnings for complications such as dehydration or atrial fibrilation.
Since the aVAD shares some design elements with the ReliantHeart’s Heart Assist 5 LVAD, the company was able to secure the CE Mark approval without going through a new clinical trial. They expect to move forward with a trial early next year, as part of the process to earn an FDA nod.




