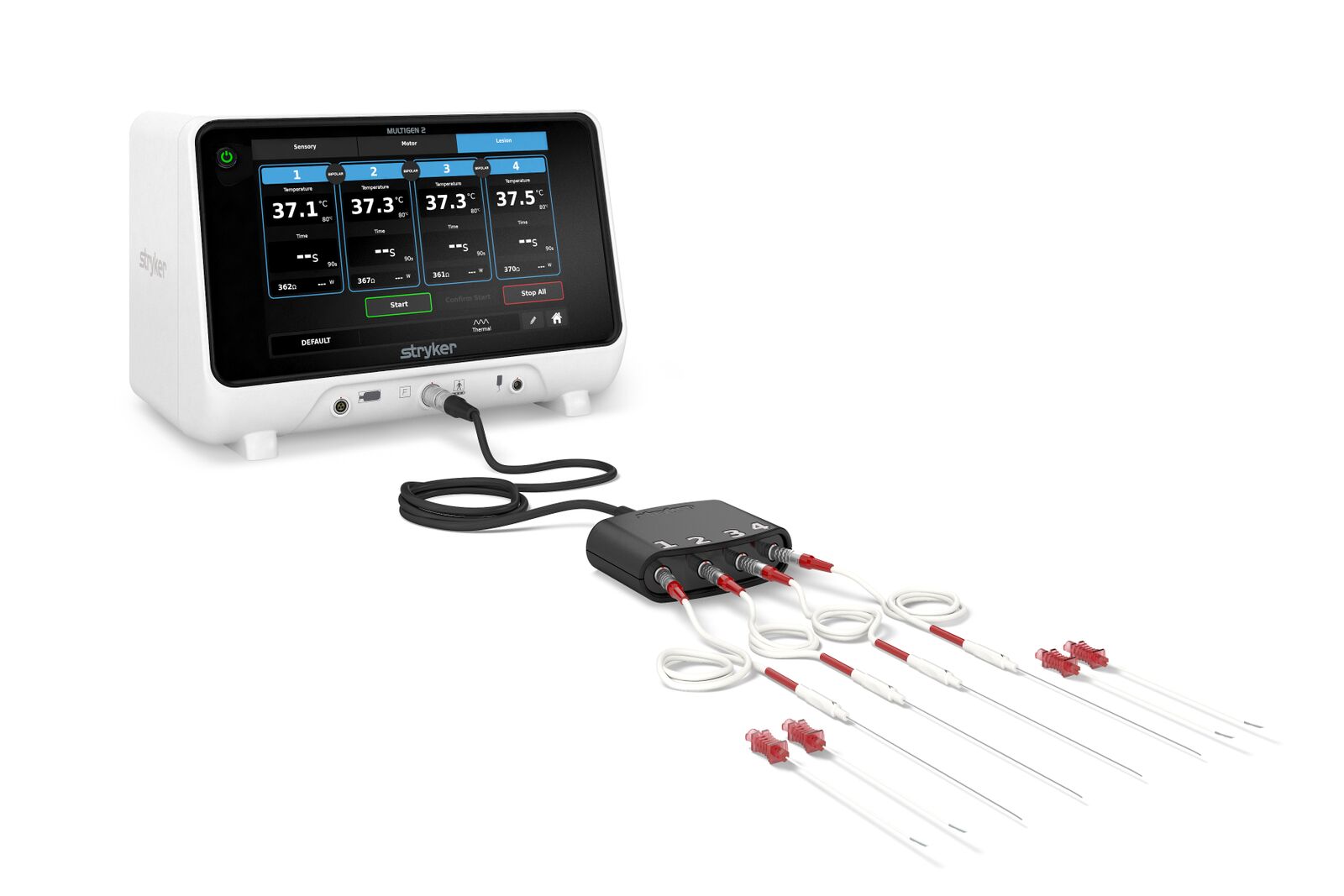
Stryker announced today that it has received FDA 510(k) clearance for its MultiGen 2 RF Generator. This product provides physicians with the efficiency, control and reliability they need when performing radiofrequency ablation, a minimally invasive procedure that can provide lasting relief to those suffering from facet joint pain.
Seventy million Americans experience low back pain at any given time. Facet joint pain is a well-recognized source of pain in patients with persistent back pain. Multiple clinical studies show that for the majority of patients, radiofrequency ablation significantly reduces pain severity and frequency for one year.
“The next generation of radiofrequency ablation has arrived,” says Brad Wallace, brand manager for Stryker. “The MultiGen 2 RF Generator provides physicians with control and confidence, making radiofrequency ablation more efficient and reliable than ever before.”
(Image credit: Stryker)
Engineered with double the industry standard for power, the MultiGen 2 Generator achieves target temperature faster, with fewer errors, for increased reliability and efficiency. Physicians can start a procedure with the push of a single button, create strip lesions without removing electrodes and resolve errors without stopping the procedure, maximizing time and schedules. Procedures are customizable based on patient needs and physician preferences with flexible stimulation controls.
The MultiGen 2 RF Generator and Venom Cannula and Electrode System make up Stryker’s Performance Platform. Physicians can confidently create the industry’s largest, least-invasive lesions in the shortest amount of time. Together, the Performance Platform delivers reliability, efficiency, and ultimate control.




