Stryker’s Spine division has announced that its Serrato Pedicle Screw, intended for use in the non-cervical spine as part of the company’s successful Xia 3 Spinal System, has received 510(k) clearance from the U.S. Food and Drug Administration.
Serrato Pedicle Screws feature enhanced serrated cutting flutes, a unique dual-thread pattern with an increased number of leads for rapid insertion, and a patented buttress thread locking mechanism designed to minimize cross threading and splaying of the screw head. The screws accommodate a variety of rod diameters and materials to suit the patient’s needs — 5.5 and 6.0mm diameter rods in commercially pure titanium, titanium alloy, and Vitallium.
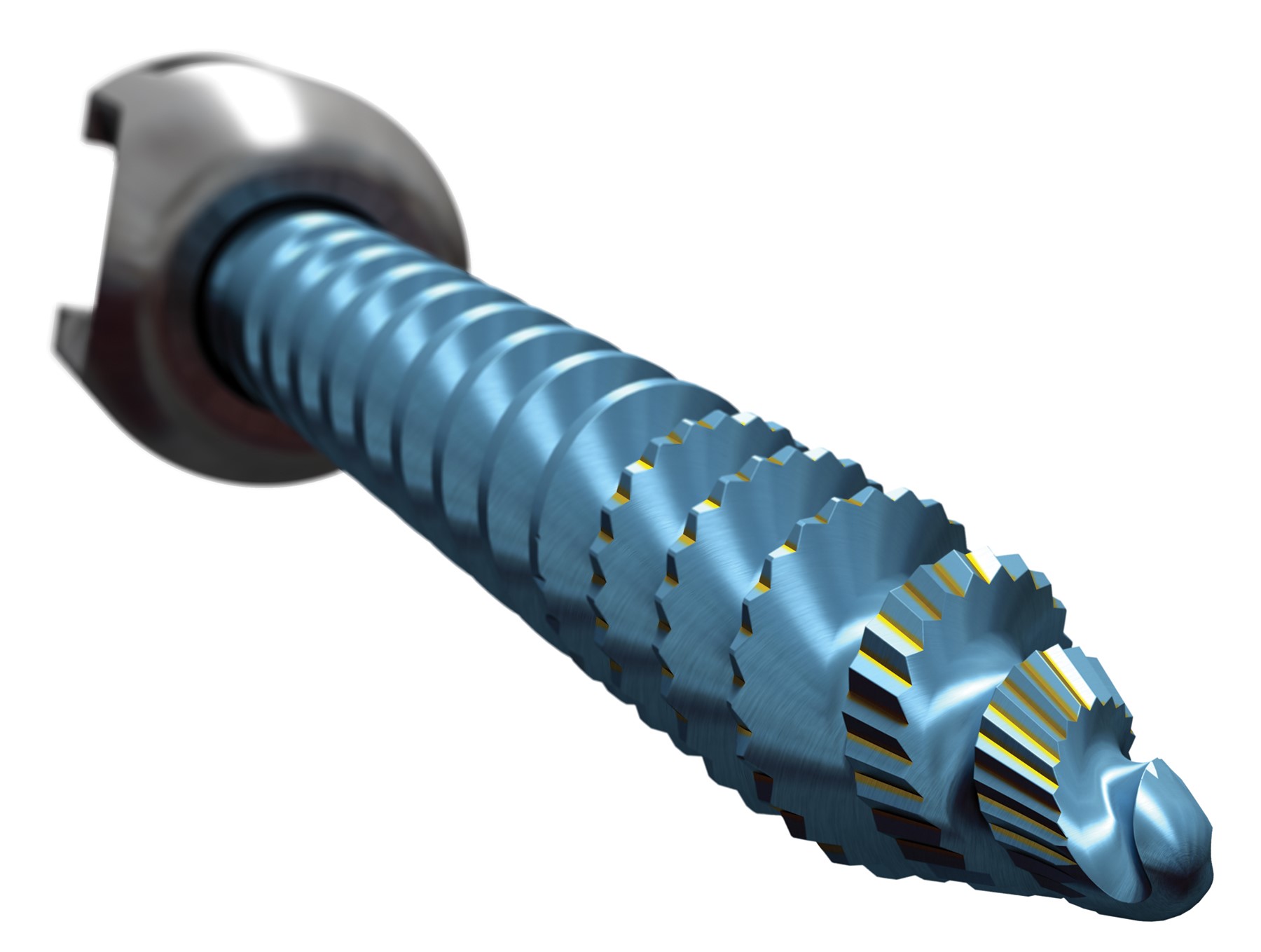
(Image credit: Stryker)
“Pedicle screws have been used for decades with very few changes to their design,” says Bradley Paddock, president of Stryker’s spine division. “The design innovations incorporated into Serrato reinforce our commitment to making industry-leading investments focused on providing the advanced spinal products and differentiated technologies that our surgeon customers have come to expect.”
Serrato leverages the broad portfolio of the Xia 3 Spinal System, an orthopaedic spinal system comprised of a variety of shapes and sizes of screws, blockers, and hooks that affix several different types of rods and connectors to vertebrae or the spinal column for purposes of stabilization, or corrective action through the application of force.




