Over the course of the past six months, Neural Analytics announced both CE Mark and FDA clearance for their inaugural device, the Lucid M1 Transcranial Doppler Ultrasound System. According to company promotional material, the Lucid M1 is meant to transform the diagnostics process for brain disorders, providing physicians with a straightforward, non-invasive method to evaluate blood flow.
To learn more about Neural Analytics and the Lucid M1, Surgical Products interviewed the company’s co-founder and CEO, Leo Petrossian, PhD.
What led you and your colleagues to start Neural Analytics?
Although we have made significant advances in extending human lifespan in the past century we have not made similar strides in extending the quality of life as we age. The lack of advanced tools to assess brain health have created a gap in care where diseases like stroke, Alzheimer’s, and dementia strip us of our ability to maintain our mental health as we grow old. It was with this perspective that Neural Analytics was founded in 2013 with the mission to improve brain health management. We are building a revolutionary technology platform for brain health assessment designed to rapidly accelerate treatment for the most significant of brain disorders.
Can you tell us about the Lucid M1 Transcranial Doppler Ultrasound System?
The Lucid M1 System is our first FDA-cleared technology and represents the foundation for our future brain management platform. This product combines medical ultrasound with the principles of the Doppler effect to non-invasively, and from outside the body, measure the health of the brain’s blood vessels and monitor for blood based particles that can potentially cause stroke.
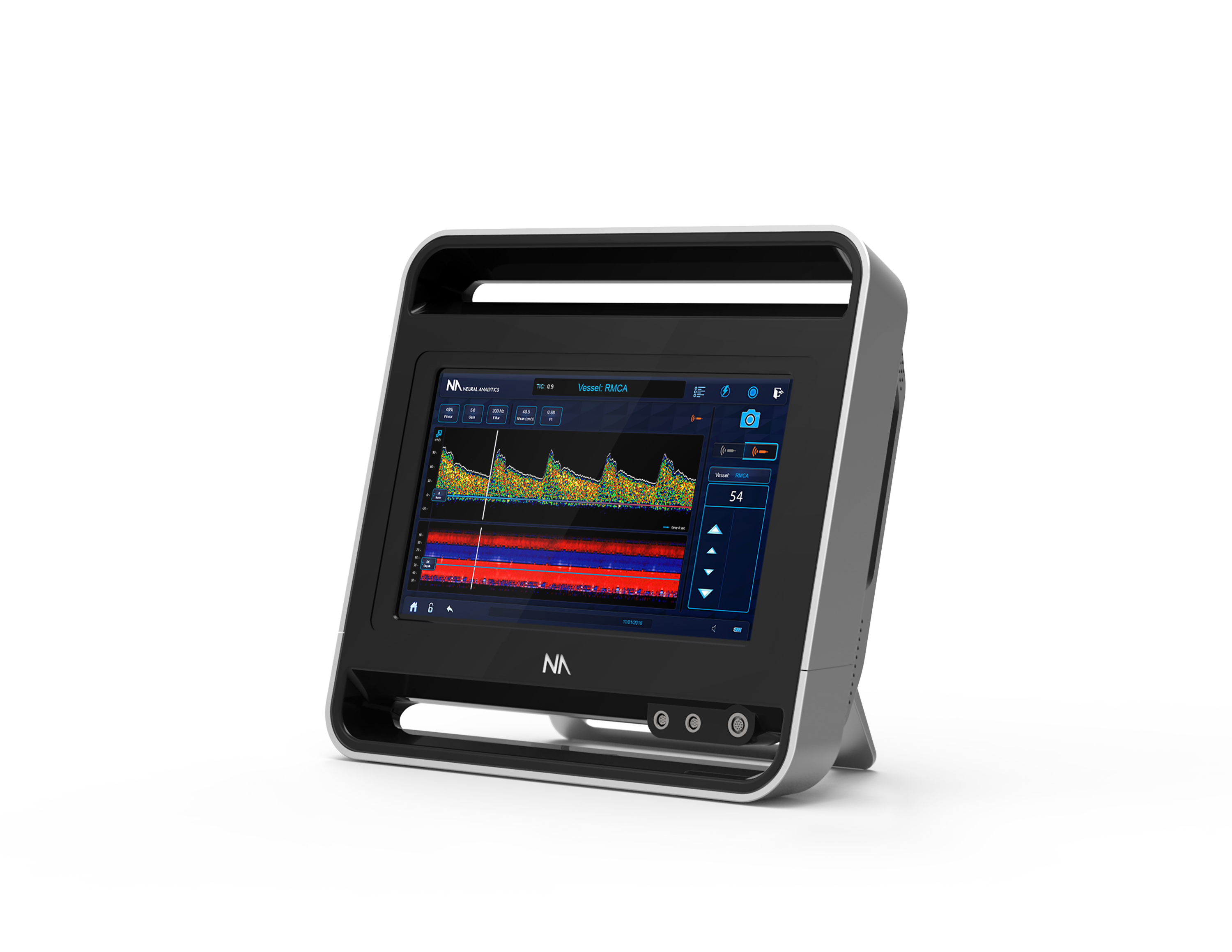
The Lucid M1 Transcranial Doppler Ultrasound System. (Image credit: Neural Analytics)
For those healthcare professionals who adopt the device, what will be the major improvements they’ll see compared to current tools and practices?
The Lucid System is a modern, post-smartphone version of a legacy technology known as transcranial Doppler ultrasound. We have worked to improve upon both the usability and the capability of the system over existing alternatives. Our system is highly intuitive, lightweight, portable, and small, all while packing in incredible power, resolution, analytics, and reporting capabilities. These significant improvements make it ideal for use in fast-paced neuro environments where users don’t have the time to haul a cart around the clinic or worry about how to use a legacy technology.
How do you envision this technology will impact brain care moving forward?
For too long, we have only had a limited understanding of the dynamics of brain health due to the limitations and availability of appropriate diagnostic tools. Our mission is to develop the future tools that will enable healthcare professionals to improve our understanding of brain health and the diagnosis of brain disorders. We hope to help pave the way for this innovation to significantly improve outcomes for patients while reducing the high costs associated with brain care.
Is there anything else you’d like to add?
This year is pivotal for Neural Analytics as we transition from an early stage medical technology company to an international commercial organization. During 2017, we anticipate several significant milestones, including expanding our clinical studies programs to include new disease states and applications in brain health, as well as building a commercial presence in regions outside the U.S. and Europe as we secure additional regulatory approvals.




