According to Select USA, there at least 6,500 medical device companies in the United States with a market side of about $148,000,000,000.00.
Medical devices include homecare devices for diabetes, pacemakers and other implants, surgical equipment, dental equipment, and more. While many devices are helpful and even life-saving, others sometimes have a negative effect on patients. Devices may stop working properly or do not last and break. Recently, St. Jude Medical called attention to a faulty battery in some implantable heart devices saying that a defect causes them to stop working early.
Here is summary of recalled class I medical products from 2015-2016, according to the FDA:
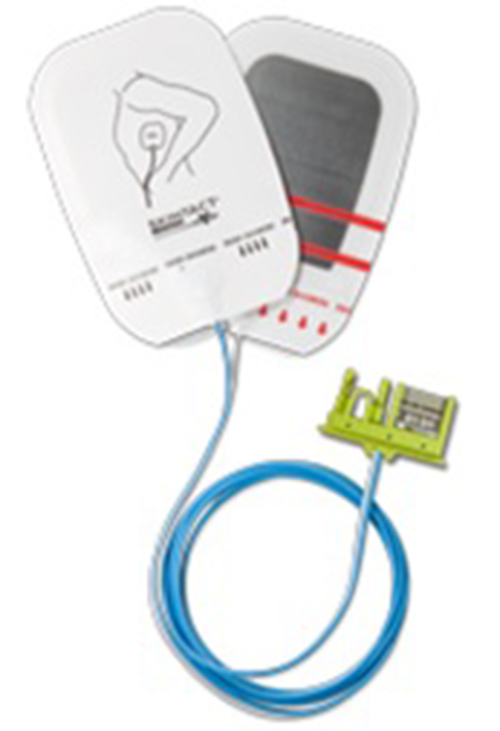
1) Recalled Product:
- 50028 Defibrillation Electrode SKINTACT DF29N
- Lot Numbers: 60602-0774, 60502-0779, 60308-0771,60114-0773, 51023-0775, 50904-0777, 50403-0778, 50130-0777, 41023-0771, 41008-0778 40730-0778, 40618-0778, 40130-0776
- Distribution Dates: February 14, 2014, to August 3, 2016
- Devices Recalled in the U.S.: 8040 nationwide
(Credit: FDA)
This Automatic External Defibrillator (AED) was recalled because of a compatibility issue it had with the Welch Allyn AED model 10. This issue would have caused the defibrillator to not shock when it was needed, resulting in potential death.
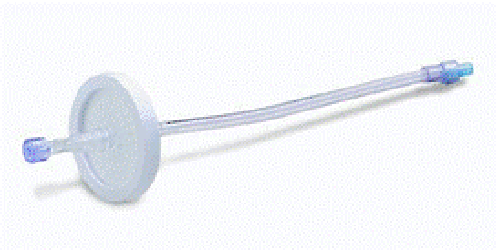
2) Recalled Product:
- 50 mm 0.2 Micron Filter
- Product code: H93835
- Lot numbers: All unexpired lots
- Manufacturing dates: June 27, 2013 to June 27, 2016
- Distribution dates: August 12, 2013 to June 20, 2016
- Devices recalled in the U.S.: 130,100 units nationwide
(Credit: FDA)
Used for bacteria filtering in pharmacies located in hospitals, this disposable micron filter helped facilitate repeatable fluid drug dosage. However, the product was recalled because of particulate matter and potentially missing layers of the filters that could lead to a contaminated solution.
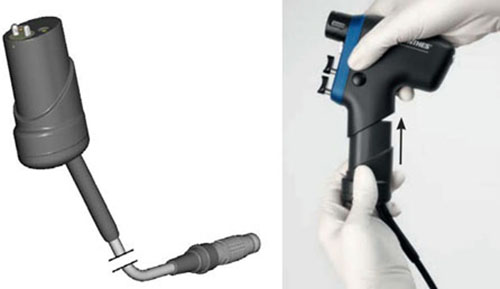
3) Recalled Product:
- Adaptor and Light Adaptor for Small Battery Drive and Small Battery Drive II
- Serial Numbers: 05.001.024 and 05.001.108
- Manufacturing Dates: October 6, 2005 to April 5, 2016
- Distribution Dates: January 2006 to June 2016
- Devices Recalled in the U.S.: 451 units distributed nationwide
(Credit: FDA)
These power sources for surgical power tools were recalled because of high internal pressure, leading to possible explosion, causing potential injury or death.




