As I mentioned in another piece I featured earlier this week, the Children’s National Health System recently announced the finalists of their Third Annual Pediatric Surgical Innovation Symposium hosted by the Sheikh Zayed Institute for Pediatric Surgical Innovation. The competition is intended to bring attention to the needs of pediatric patients in having medical technology developed specifically for them and to bring about actual innovations for that effort. The two winning teams of the eight finalists will be announced this Friday and will each be awarded $50,000.
While the following interview represents the second team to have their submission to the pediatric-focused technology contest highlighted, it’s actually a device that can be used for patients at any age level. Due to intubation devices being developed for the size of an adult patient, however, this technology is best suited for use with infants and children.
Another aspect of this interview that I particularly liked was it reflects a case of a doctor seeing a clinical need and going ahead with the effort of attempting to take part in the solution. Dr. Donald S. Prough is the chair for the Department of Anesthesiology at The University of Texas Medical Branch. He observed a problem with current technologies for the intubation. Currently, Dr. Prough is also serving as the interim CEO of the company behind this intubation technology, which he offers more details about in the interview.
Sean Fenske: Please briefly explain your technology and how it can affect healthcare.
Dr. Donald S. Prough: Prospiria has developed a patent-pending technology that uses optoacoustics (ultrasound signals generated by pulsed laser light) to rapidly, precisely, and noninvasively identify the position within the trachea of endotracheal tubes that have been placed for temporary mechanical support of breathing. Maintaining better positioning of endotracheal tubes will limit severe complications related to tubes that are inserted too far, which could potentially lead to lung collapse and inadequate oxygenation, or insufficiently far, which could potentially lead to accidental extubation.
Fenske: How does it work?
Dr. Prough: Pulsed laser light that is emitted from an optical fiber within an endotracheal tube penetrates the tissues of the neck and generates an ultrasound signal that is detected by an ultrasound detector on the surface of the neck.
Fenske: What was the inspiration for developing it?
Dr. Prough: As an anesthesiologist who is involved in clinical care and research on infants and children, a challenge that made an impression on me was the recurring problem caregivers have in knowing both the initial position of an endotracheal tube immediately after placement and recognizing changes in position that inevitably occur in the course of care. The best available diagnostic test for determining endotracheal tube position is a portable chest X-ray, which exposes the patient and the caregivers to radiation, requires considerable time, provides only a one-time snapshot of endotracheal tube position and necessitates patient movement, which could itself cause accidental extubation.
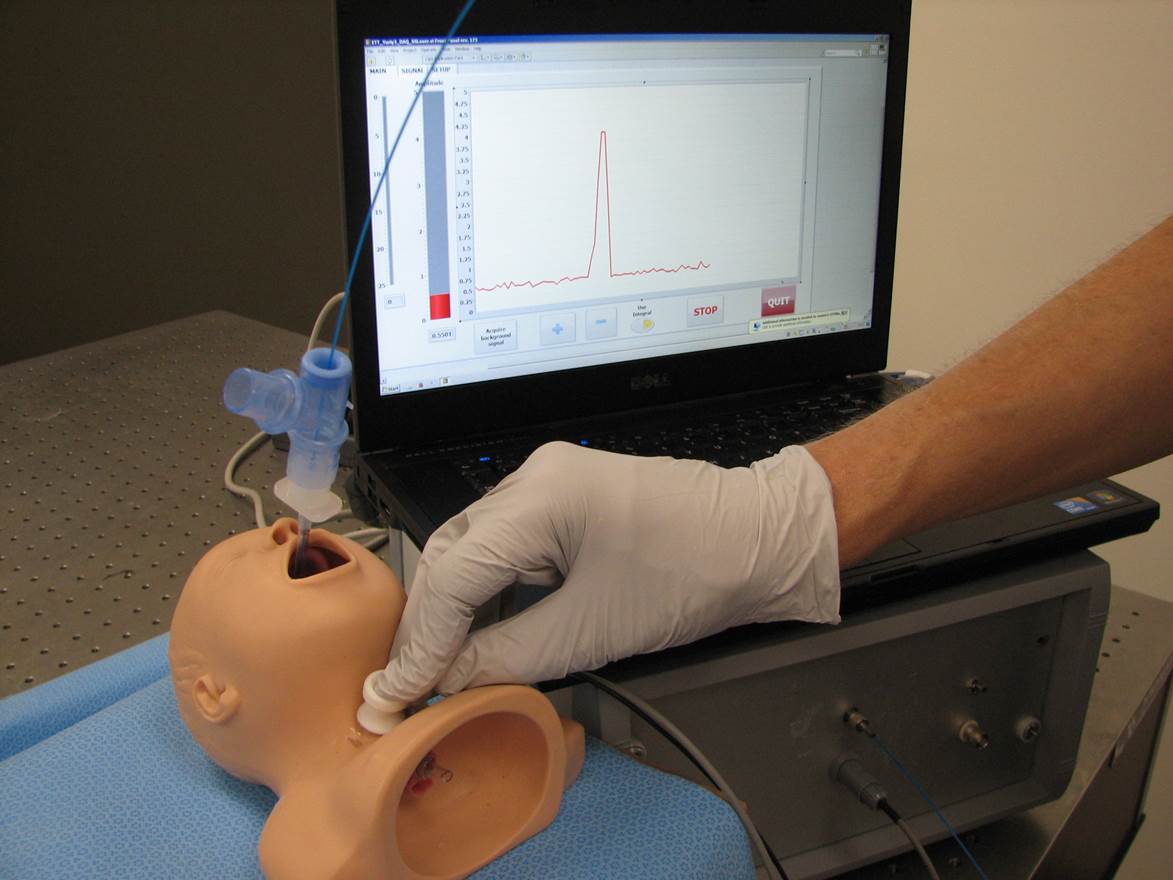
The clinical prototype of the Prospiria intubation detection technology
Fenske: What challenges have you encountered with regard to development?
Dr. Prough: We have encountered few major challenges. Proof-of-concept and proof-of-principle studies proceeded rapidly and we quickly developed a clinical prototype with which we have conducted clinical studies in infants, children, and adults. The major upcoming challenge is to build a manufacturing prototype to submit for clearance by the Food and Drug Administration (FDA).
Fenske: Have you explored the regulatory aspect of medical device development yet?
Dr. Prough: We have not yet met with the FDA. However, this is a minimal risk device for which there are likely antecedent devices, such as light wands and portable ultrasound machines. Therefore, we anticipate a 510k device exemption.
Fenske: What’s the most significant thing you’ve learned about medical device development during this experience?
Dr. Prough: The most striking aspect for me of medical device development is the magnitude of the obstacles to continued development, even of technology that is straightforward to build and use.
Fenske: Why did you enter the Annual Pediatric Surgical Innovation competition?
Dr. Prough: Although this medical device can be used in adults as well as in infants and children, the greatest need for the technology is in infants and children because of the short length of their tracheas relative to the length of endotracheal tubes. An airway problem in an infant or child necessitates prompt and effective recognition and intervention.
Fenske: What have been your thoughts of the competition experience so far?
Dr. Prough: I also participated in the competition last year and was highly impressed with the quality and range of the competing technologies. The conference was well organized last year and the submission process both this year and last year was efficient. The organizers maintain excellent communication with the participants.
Fenske: After the competition, what’s next for your company?
Dr. Prough: Our next steps are to identify a CEO with business experience and expertise to lead Prospiria in fundraising so that we can progress to meeting with the FDA, building a manufacturing prototype, conducting clinical studies with that prototype, and obtaining FDA clearance.




