FundamentalVR, pioneers of immersive training technology for the surgical community, has announced its platform Fundamental Surgery, which combines virtual reality with haptics (the sense of touch), has received Continuing Professional Development (CPD) accreditation by the Royal College of Surgeons of England. It is the first surgical simulation with HapticVR™ to receive CPD points and covers the Fundamental Surgery Total Hip Replacement (Posterior Approach) training simulation
CPD is set by General Medical Council (GMC) as activity outside of postgraduate training that demonstrates surgeons are continuing to improve and practice their skills and performance. The Royal College of Surgeons of England accredits activities ranging from educational days and conferences that are in line with the GMC’s guidelines and the number of points each activity counts towards each trainee’s annual CPD points.
To maintain professional standards, surgeons must accrue 50 CPD points a year (250 over a 5 year revalidation cycle). With this accreditation, Fundamental Surgery’s simulation is confirmed as an activity that demonstrates sufficient educational value to contribute to the CPD for which 6 CPD points can be claimed.
Named as one of the best inventions of 2018 by Time magazine, the Fundamental Surgery platform was launched in August 2018. It combines virtual reality (VR) with cutting-edge haptics to create a scalable ‘flight simulator’ experience for trainee and qualified surgeons, allowing them to experience and navigate the same visuals, sounds and feelings they would during a real surgical procedure. What sets Fundamental Surgery apart from other solutions is that it is designed to be equipment agnostic, compatible with any laptop, VR headset or haptic device enabling it to be delivered at a fraction of the cost. Furthermore its remote data analytics and data dashboard covering surgical skills and knowledge provide invaluable insight into surgical capability and education progression.
While other simulations are limited to visual and audio interactions, Fundamental Surgery takes it to a new level with HapticVRTM, its proprietary technology that adds a real-time sense of touch. Surgical trainees can feel the movement and interaction of tissue, muscle and bone as they would in an actual procedure within a submillimeter of accuracy of resistance. Fundamental Surgery has a library of tools and tissue variants that mimic real life sensations that have been calibrated by a leading team of surgeons and KOLs.
“We are pleased Fundamental Surgery has been recognized by the Royal College of Surgeons of England as an educational platform that can help increase proficiency and help maintain and improve surgeon’s performance,” said Richard Vincent, CEO and Founder of FundamentalVR. “With Fundamental Surgery, we have developed a completely safe and realistic teaching environment for surgeons to learn and hone their skills combining virtual reality with tactile feedback that is so important for developing the muscle memory associated with different procedures.”
The platform currently supports a range of orthopedic procedures, including the Total Hip Replacement Posterior Approach (P-THR) simulation that received the CPD accreditation. The P-THR simulation supports users in maintaining and developing their understanding of relevant anatomy, pre-operative planning and post-operative patient care, as well as offering a haptically enabled simulation experience and intra-operative decision making.
Omar Sabri, Consultant Surgeon in Trauma & Orthopedics with a special interest in Pelvic reconstruction and Lower Limb arthroplastys and member of the FundamentalVR Global Medical Board said, “This is a huge step forward for VR surgical simulation and a validation of its role and propensity to add value to the surgical education system. As the first HapticVRTM simulation company to be accredited by the RCS of England for Total Hip Replacement (Posterior Approach), Fundamental Surgery is an incontrovertible leader in the field.”
The Fundamental Surgery platform is currently deployed in a number of different medical institutions. Most recently, leading London hospital, St George’s Hospital, deployed Fundamental Surgery within their simulation center. This installation enables anytime access for medical trainees to use the education platform, to monitor progress and help individual users continue to grow their skill sets. Fundamental Surgery is also deployed at the Mayo Clinic and UCLA in the US, UCLH in the UK and Sana in Germany.
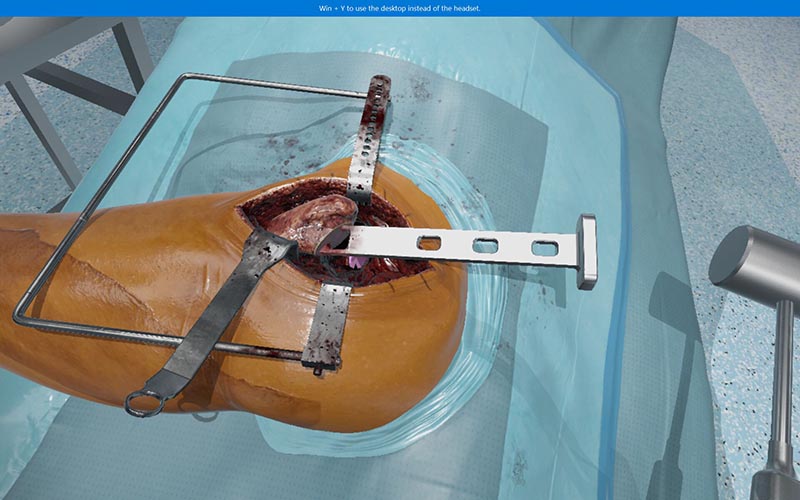
Featuring FundamentalVR’s total hip arthroplasty surgery




