Zimmer Biomet, a global leader in musculoskeletal healthcare, has announced the completion of the first surgical case utilizing its Persona TM Trabecular Metal Tibia by Dr. Richard Moore, Boise, Idaho on March 20, 2018. The Persona Trabecular Metal Tibia received 510(k) clearance from the FDA in January 2018 and CE Mark approval in April 2018. The Persona Tibia is an integral component of the Company’s portfolio of cementless total knee arthroplasty (TKA) solutions.
“Combined with the Persona TM Femur and the TM Patella, clearance of the Persona TM Tibia allows us to provide a fully cementless total knee solution, furthering our long-standing commitment to enhancing patient experiences and outcomes,” said Dan Williamson, Zimmer Biomet’s Group President, Joint Reconstruction. “We believe this innovative product will position us to re-establish our leadership in the fully cementless primary knee market.”
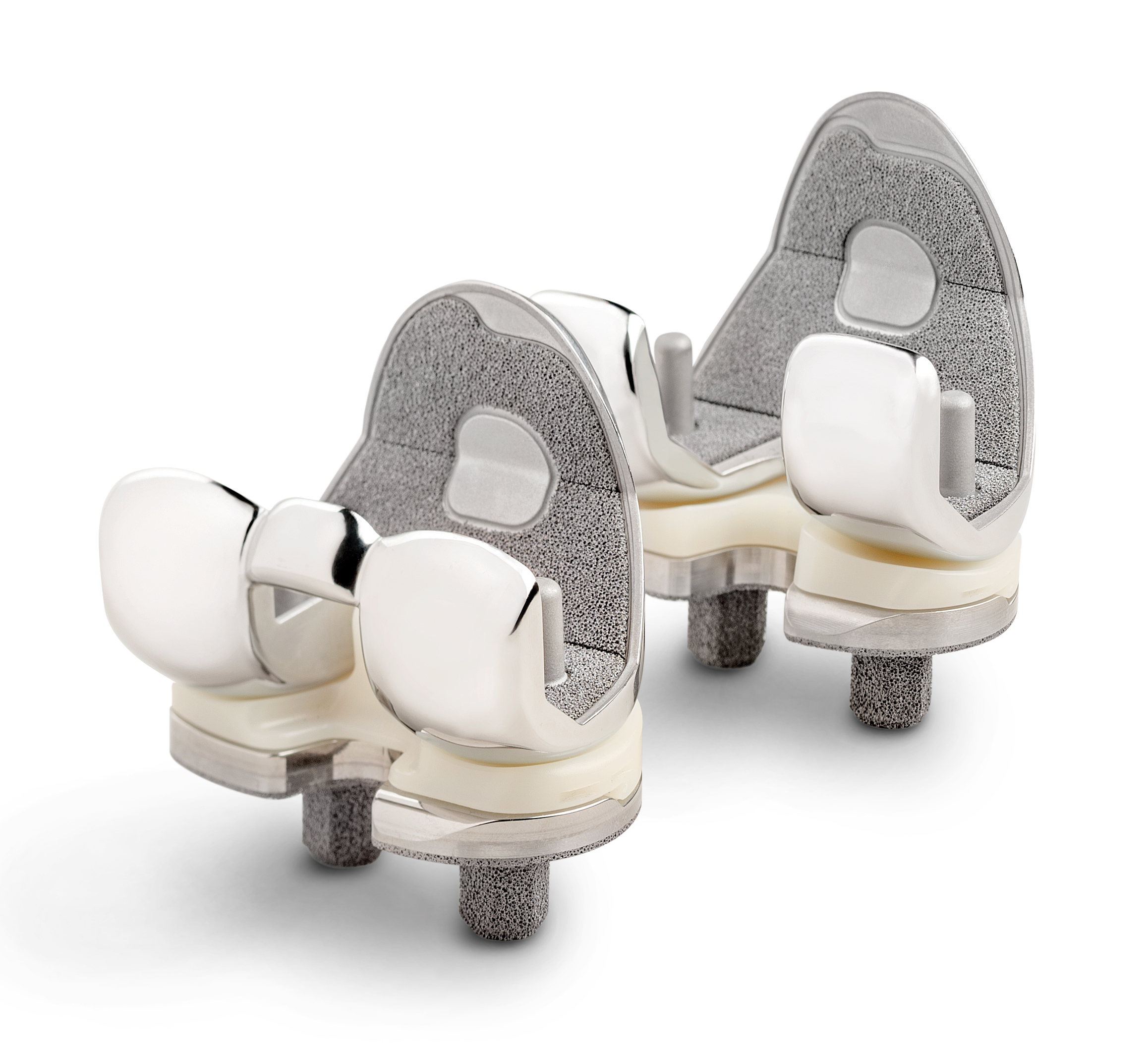
Zimmer Biomet’s proprietary TM Material is a porous biomaterial made from elemental Tantalum with structural, functional and physiological properties similar to cancellous bone.1-3 With more than 20 years of clinical results, the Company’s TM Material has been used in over two million orthopaedic devices.
(Image credit: Zimmer Biomet)
“The clearance of the Persona TM Tibia represents a significant step forward for those patients who can benefit from completely cementless total knee replacements that better integrate into the natural bone anatomy for durability, while possibly offering greater patient satisfaction,” says Moore.
The Persona TM Tibia features new drilling, sizing and insertion instrumentation that are unique to the Persona TM Tibia, while preserving all the anatomic benefits of the Persona Tibia design.4-8 The Persona TM Tibia is another example of how the Persona System continues to redefine personalization by combining Zimmer Biomet’s 20-year porous fixation expertise with the Persona Knee family. As the Company’s most comprehensive knee system, the Persona System offers more anatomically accurate components with finer increments to personalize patient fit and restore the unique identity of every knee.
“The TM tibial tray and pegs fit beautifully to the bone. I believe the instruments for trial prep and implant placement were a clear improvement and an advantage. I also appreciate the time efficiency of using cementless implants,” says Moore.
Zimmer Biomet plans a limited launch of the Persona TM Tibia in the first half of this year, followed by a full commercial launch in the second half of 2018.




