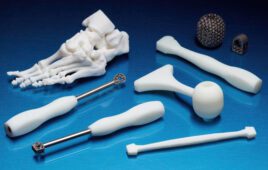
Think of Carbon’s 3D printing technology enabling a host of customized medical devices and parts. Here’s how.

Carbon’s proprietary 3D printing technology combines light and oxygen to rapidly make products from programmable and biocompatible liquid resins. [Image courtesy of Carbon]
The 2015 event generated a lot of buzz because it overcame perceptions that 3D printing was a slow, cumbersome process. And Redwood City, Calif.–based Carbon has made significant strides since then, including a $200 million fundraise earlier this year that included Johnson & Johnson Innovation.
Johnson & Johnson, in fact, has a more than two-year-old strategic collaboration with Carbon to produce customized orthopedic surgical instruments and other medical devices.
So how is Carbon’s 3D printing tech potentially better, and what does that mean for medical devices?
Steven Pollack, an FDA veteran who joined Carbon as a senior staff research scientist in 2015, thinks Carbon’s advantages rest on three pillars:
1. Carbon figured out how to make stereolithography work
Carbon’s roots go back to the University of North Carolina at Chapel Hill, where DeSimone and his colleagues tried to overcome 3D printing’s speed and quality problems. A potential solution to the layer-by-layer tediousness of other 3D printing methods was to create a part through stereolithography, using light to convert a photocurable resin into a solid.
“You do it by projecting a series of images that end up being the cross sections moving from the bottom to the top of the object and placing the next amount of material on the previous one that you created. And the big shortcoming for that is that you either have a very large vat of resin that you hold your part down into as you build parts, or you have a very shallow pool of resin that you hold your part out of. But you suffer from the problem in that latter version that your part is continually stuck to the bottom,” Pollack explained to Medical Design & Outsourcing earlier this year.
DeSimone and his colleagues realized that they could take advantage of the fact that oxygen frustrates the polymerization process, something that researchers had previously found to be a nuisance.
Carbon’s original intellectual property involves a special window at the bottom of the resin reservoir, Pollack said. The window is transparent to UV light, but it’s also permeable to oxygen. Carbon’s technology drives oxygen intentionally into the very lowest part of the resin pool, saturating the first 30 to 40 microns with oxygen and creating a “dead zone” where polymerization doesn’t take place.
“So now when you start to grow your part, you’re never growing it at the window, you’re growing it 30 microns away from it. And when you pull the part away from the window to introduce the next step of the process, the part acts like a pump, pulls fresh resin in behind it, which dilutes out the oxygen and makes that material polymerize,” Pollack said.
The result is fast printing with little or no mechanical impact on the growing part, according to Pollack.
“In our technology, there are no layers. If you broke a part in half, you’d find it was perfectly monolithic. … We think … mechanical-properties-wise, it is comparable to an injection-molded part. And surface-texture-wise, it’s comparable to an injection-molded part,” Pollack said.
2. Using production-worthy materials
When it comes to polymers that only photochemically harden, they’re unable to match the mechanical properties of, say, a nylon or an elastomer, according to Pollack. When it comes to the few UV-only resins used on Carbon machines, you can get a “nice part that’s easy to look at but not necessarily mechanically robust.”
Carbon’s solution to the materials challenge was to turn to a host of thermally-activated polymers.
“You use the build process to set up a scaffolding, if you will, that’s photochemically generated, that holds the part’s shape and accuracy. You take that part, clean it of any residual resins, and then you bake it — much like you would take a porcelain (pot) that was thrown on a wheel and put it into a furnace to fire off the secondary chemistry. … It’s the same thing. We use epoxy chemistry, cyanate ester chemistry, and urethane chemistry to create this second network that imbues the part now with real engineering properties,” Pollack said.
“We can make a material that’s equivalent to an injection-molded urethane, or a cast urethane, or an injection-molded nylon.”
3. Software-driven 3D printing technology
Carbon’s VP of engineering, Craig Carlson, came from Tesla Motors, where he was VP of software and electrical integration. “He came to Carbon with the mindset that we can capture every detail of the manufacturing and operation process of the printer and have that captured and codified, and that’ll be very important if anybody wants to know about the history of the part,” Pollack said.
Think data about the provenance of the part, what resin it came from, which printer was it on, how was that printer behaving that day, the characteristics of a particular layer.
“We capture every piece of that data, and we have it all available for every part that’s printed. It’s available to the manufacturer to create a digital master device record — a manufacturing device record,” Pollack said.
Carbon’s printers are Internet-connected, so the company is able to collect performance information and continually improve the machines through software upgrades.
“That said, we also recognize the medical device manufacturing world does not like software that upgrades every six weeks, because they then have to revalidate and verify. So we have a model that lets us lock essentially the software branch for manufacturing and production,” Pollack said.
Manufacturers don’t own the printer; they have a subscription for it.
“They keep their intellectual property on their side of the firewall. We don’t get to see the actual object they’re printing. We just get to monitor the machine’s health,” Pollack said.
One of Pollack’s major jobs at Carbon is to work with his old colleagues at FDA to shepherd the new materials and processes through medical device approval and clearance processes, and Carbon’s technology has some regulatory challenges to overcome. But Pollack thinks it could eventually prove disruptive.
“You can now throw away the molds, throw away the inventory,” Pollack said. “You can throw away the notion that, ‘Oh, I have to make that part from seven pieces and glue them together so I can get channels to go through them.’ … I put negative space where I want it as I build it. I don’t have to take things away to get it there. It’s the difference between subtraction and addition.”