Carbon, the 3D printing company, announced today that it is working with Adidas on face shields for healthcare workers and with Resolution Medical on nasal swabs for COVID-19 testing.
Carbon, the 3D printing company, announced today that it is working with Adidas on face shields for healthcare workers and with Resolution Medical on nasal swabs for COVID-19 testing.
Adidas tweeted about its work with longtime collaborator Carbon, saying the athleticwear company is aiding Carbon’s production of face shields, which healthcare providers are using to protect themselves from contracting the virus.

(Image from Resolution Medical)
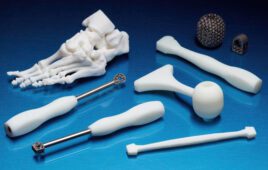
In vitro diagnostic and medtech manufacturer Resolution Medical (Minneapolis) announced the launch of its medical lattice swab, made using Carbon digital light synthesis technology and Keystone Industries’ KeySplint Soft Clear material for Carbon printers. The material is indicated for the fabrication of orthodontic and dental appliances such as mouthguards, night guards, and snoring appliances in the US, Canada, EU, Australia, and New Zealand.
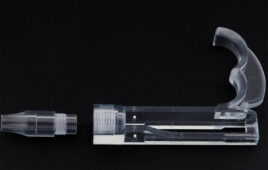
Reports of swab shortages have been coming in from across the country, according to a report by Bloomberg News. Another 3D printing company, Formlabs (Boston) said it is working to supply hospitals with 3D printed COVID-19 test swabs.

(Image from Formlabs)
Formlabs said it is mobilizing its community of users to deploy nearly 1,000 printers to quickly mass-produce these swabs as well as other important personal protective equipment (PPE). A single print can produce 300 test swabs at a time, enabling Formlabs to produce 75,000-150,000 swabs per day.The Formlabs team is working with three leading U.S. hospitals, as well as Boston-based medical professor Dr. Ramy Arnaout on the swab design. The company plans to print these swabs in-house and share the design files with its community as well as other health systems to scale the project nationwide.
Resolution Medical said it has already begun shipping its FDA 510(k)-exempt nasopharyngeal swabs for COVID-19 diagnostic testing to healthcare organizations and is able to provide swabs to others in the U.S.
The hollow structure of the lattice is designed for specimen collection efficiency, with a flexible geometry to promote its function and patient comfort, according to Resolution Medical. Clinicians at multiple institutions, including Beth Israel Deaconess Medical Center, a Boston teaching hospital affiliated with Harvard Medical School, and Stanford Medicine, are evaluating the swabs.
“We are proud to be collaborating with digital manufacturing company, Carbon, to produce the lattice swab,” said Resolution Medical president Shawn Patterson in a news release. “We have worked together urgently to get this product into the hands of healthcare workers to help address immediate needs for increased COVID-19 testing. At scale, we plan to supply over 1 million swabs per week.”
The company is working with Carbon’s network of dental labs and production partners, as well as its internal printing team. The swabs are biocompatible and autoclavable and printed hundreds at a time with a unique serial number on each strip to make it traceable.
“Triggered by the COVID-19 pandemic, Carbon’s engineers and material scientists quickly sprung to action to identify the KeySplint Soft Clear material as having the right balance of properties to make a soft, flexible swab with appropriate strength that could be printed with precision using the Carbon M2 at 75 micron pixels,” said Carbon executive chairman Dr. Joseph DeSimone. “Resolution Medical, our production partner since 2018, has been amazing in leading the effort to launch the product.”
This article has been updated with information from Formlabs.