
[Image from C&K]
The new medical switches are designed to meet industry sterilization standards that protect patients from risks that comes from reusable tools. Sterilization helps prevent contamination from fluid and contagion ingress.
“Medical device engineers are creating the applications that people entrust to keep them safe – from diagnostic equipment and active minimally invasive surgical tools to eHealth products – and it’s important that the components that make up these devices don’t fail when needed most,” said Roger Bohannan, medical segment leader at C&K. “C&K’s new medical switch product line is ideal for designers creating the quality, superior performing devices that the medical community relies upon to work properly in the most challenging situations.”
C&K’s new medical switch product line has IP67 seal switches that have been tested for biocompatibility and are ideal for medical devices that require a sterilization process step. They are also autoclave safe, gamma sterilization compatible and EtO sterilization safe.
The new KSC series feature autoclave safe switches that are designed for medical devices that have to withstand chaotic medical environments like the emergency room. They can last up to five million cycles and the silicon actuators with with switch integration.
KMR microminiature SMT tactile switches are designed for a variety of harsh environment applications. The compact tact, top-actuated switches come with Gullwing termination and have four available actuation forces.
C&K’s new K12 series dual action switch enables customization options. The different configurations can reduce the number of actions required for use to help medical professionals finish procedures in fewer steps.




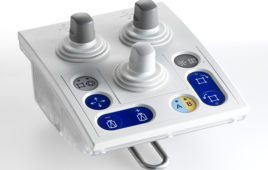
IP levels of protection is critically important to the Drug Delivery Systems that are being used in today’s connected health environment. They protect medical devices that have a shelf life prior to use and well as harsh environments while in use. C&K engineering and R&D is releasing needed products for the Medical Device Industry. We are entering a very interesting time as more and more medical devices require active components for data transmission, dosage tracking and contact confirmation.
If you need to turn a medical device on, transmit data or confirm contact, C&K is there to help with a medical grade swtich sized, designed and manufactured to meet the demands of the competitive medical industry market. Contact us. CKSwitches.com