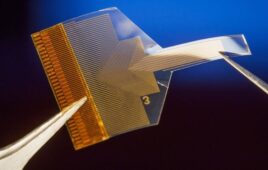
The IDT sensing solution incorporates an LED driver, temperature sensor, photodiodes and other components in a compact package to drive glucose measurements, which are wirelessly communicated to an on-body wearable transmitter. Image source: www.todaysmedicaldevelopments.com
Integrated Device Technology announced that an implantable long-term glucose sensor using IDT’s sensing technology has received government approval to be marketed in European Union (EU) member countries.
Maryland-based Senseonics received the CE Mark approval for its Eversense Continuous Glucose Monitoring (CGM) System, featuring an implanted glucose sensor that lasts up to 90 days or about six times longer than non-implantable systems currently on the market.
The IDT sensing solution incorporates an LED driver, temperature sensor, photodiodes and other components in a compact package to drive glucose measurements, which are wirelessly communicated to an on-body wearable transmitter.
In developing the semiconductor for Senseonics, IDT engineers selected a technology usually used for hearing aids that can be operated at a voltage as low as 0.85 V. The biotech company Roche announced last month that it will market the Eversense CGM System in Italy, Germany and the Netherlands.
Integrated Device Technology
www.idt.com