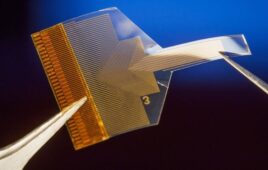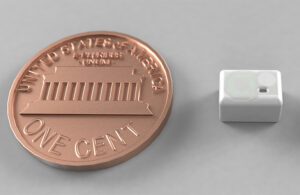
A rendering of Lura Health’s saliva sensor with a U.S. penny for scale [Image courtesy of Lura Health]
The company aims to start by noninvasively measuring saliva acidity to help prevent tooth decay, which is the most prevalent health condition globally and the most common chronic disease in children and young adults.
“It affects at some point around 90% of Americans,” Lura Health co-founder and CEO Daniel Weinstein said in an interview with Medical Design & Outsourcing. “There’s a population set for which it imposes a huge burden. And dental expenditures are $162 billion or more in the U.S. alone, so it’s a big economic and health toll.”
Weinstein founded the company in 2017 with Chief Medical Officer Dr. Saam Bozorg and Chief Technology Officer Noah Hill, all of whom were studying at Tufts University at the time. Bozorg was a dental student, Hill was studying embedded systems computer science engineering, and Weinstein was working toward his degree in bioengineering and biomedical engineering. They secured their first funding from university competitions and started working on a prototype.
All these years later, Lura Health plans to submit for FDA de novo classification in late 2024, and after winning regulatory approval, the company would file for expanded indications to include measuring glucose for diabetes, as well as electrolytes and metabolic panel elements for kidney and heart disease.

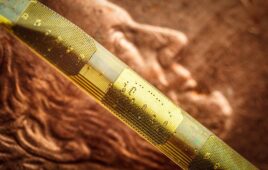
This rendering shows Lura Health’s saliva sensor in a retainer [Image courtesy of Lura Health]
“We’ve created a black box that can be integrated into most if not all dental products that people are already getting. It adds smart features to that existing distribution.”

Lura Health’s saliva sensor can wirelessly recharge in a carrying case. [Image courtesy of Lura Health}
Resonant Link developed an ultra-low profile design for the oral implant sensor to maximize energy transmission while charging for speed and temperature control.
“We were able to achieve an extremely tight coupled charging, which also is great because it doesn’t generate heat,” Weinstein said. “It meets our FDA requirements, all extremely localized in the mouth.”
Medication monitoring
The startup’s ultimate market segment will be medication monitoring, Weinstein said. The technology could provide real-time, continual feedback to track therapeutic windows of medications and track targets to ensure correct dosage, compliance and effectiveness.
“We can hopefully do that at home rather than having the patient come into the clinic and getting all these draws,” Weinstein said. “And it can save everyone a lot of money, convenience, and ultimately be better.”
Lura Health’s sensors could start measuring medication soon through partnerships with pharmaceutical developers under the right circumstances.

Lura Health co-founder and CEO Daniel Weinstein [Photo courtesy of Lura Health]
“That’s the road map. We think we can get a lot of meaningful data sets,” Weinstein said. “… There’s a lot we can do even before our FDA submission.”