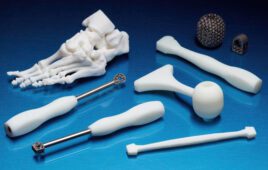
This flexibility is what intrigues those in the medical device industry to use 3D printing for orthopedic and cranial implants, surgical instruments, dental restorations, prosthetics and other devices.
Editor’s Note: This article is written by Kate Keverline, a writer from Regulatory and Quality Solutions LLC.
Just this week the world’s first 3D-printed office opened in Dubai. It took all of 17 days to print a 2,700-square-foot building at half the price of conventional methods. In short, this 3D printing thing has some legs, and we mean that literally, too.
3D printing is definitely a hot topic across the medical device industry. This ground-breaking technology that began in the 80s has advanced into a valuable tool for manufacturers, and even earned itself draft guidance from the FDA earlier this month.
The basics
Also known as additive manufacturing, 3D printing creates three-dimensional figures by building successive layers of raw material. Objects can be produced from digital files, such as MRI images or CAD drawings. In this way, manufactuers and designers are easily able to make design changes or cater to a patient’s specific anatomy. This flexibility is what intrigues those in the medical device industry to use 3D printing for orthopedic and cranial implants, surgical instruments, dental restorations, prosthetics and other devices.
How the FDA sees it
The FDA’s 25-page draft covers the entire 3D print process from design to testing, and provides recommendations for proper testing and marketing of the device. Manufacturers must also clearly iterate each step in the printing process to ensure the FDA’s desired consistant end result, the draft explains.
With the guidelines approval, manufacturers will have to fulfill QMS requirements in order to obtain an application for premarket approval, 510(k) submission or humanitarian device exemption. The process could get a little tricky, though, in the case of 3D printing of biological or cellular products.
Many and more specific details can be found on the FDA website. Regulators are contiuing to take advice and suggestions on the document over the next few months. See all 28 pages of the full draft guidance here.
Regulatory and Quality Solutions LLC
www.rqteam.com