Regulatory, Reimbursement, Standards and IP
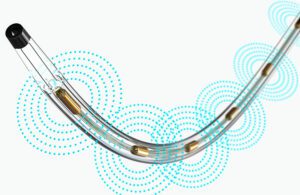
Real-world evidence can help device developers innovate with new or improved designs. Pictured is Boston Scientific’s EkoSonic Endovascular System (EKOS), which uses ultrasound energy combined with a thrombolytic drug to treat blood clots. [Image courtesy of Boston Scientific]
Real-world evidence (RWE) is a transformational concept for device design, says the chief medical officer of Boston Scientific’s Peripheral Interventions business.
Medical Design & Outsourcing: Boston Scientific — 2023
J&J used RWE for expanded indications — and you can, too
Two J&J MedTech leaders shared advice to help medical device developers use real-world evidence (RWE) in FDA submissions.
Medical Design & Outsourcing: J&J MedTech’s Biosense Webster — 2023
Tips to help device developers get paid from smart ortho implant maker Canary Medical
Canary Medical founder and CEO Dr. Bill Hunter offers his advice for demonstrating value to regulators, payors, doctors and even patients.
Medical Design & Outsourcing: Canary Medical — 2023
Why linking clinical trials to real-world data is the critical next step for medical device development
Connecting medical device clinical trials to real-world data (RWD) helps demonstrate efficacy, safety and cost-effectiveness.
Medidata — 2023

Compliance with the EU’s MDR often requires multidisciplinary collaboration between a device maker’s operations, R&D, regulatory and quality teams, plus partners across the supply chain. [Photo courtesy of Avery Dennison Medical]
With the Medical Device Regulation in effect, European Union regulatory compliance requires rigorous attention and cross-functional collaboration.
Avery Dennison Medical – 2022
What does the future hold for AI in medical devices?
Industry experts offer their perspectives on regulatory and reimbursement issues for artificial intelligence in medtech.
Pear Therapeutics, FDA, Hogan Lovells and Johnson & Johnson – 2022
Can I get IP for my healthtech AI?
With healthtech software investment booming, companies are seeking guidance on when and how to protect that work.
Greenberg Traurig – 2022
Staying on the compliant side of change control regulations
Medical device change control regulations have a lot of boxes to check.
MasterControl – 2022

[Image from Unsplash]
The success of remote clinical trial oversight opens the door to hybrid approaches and creates new possibilities for the future of trials.
DeviceTalks/Avania – 2021
Keys to protecting your medtech AI from competitors
AI is a hot area in medtech. A panel of intellectual property experts had advice on protecting the IP.
DeviceTalks/Finnegan – 2021
3 questions medical device manufacturers should ask before ISO 10993-17 updates
The upcoming publication of ISO 10993-17 will support the latest ISO 10993 expectations already in place. Gain insights into what this standard will mean to the evaluation process and how to prepare in advance.
WuXi AppTec Medical Device Testing – 2021
Introducing ‘BYOP’ clinical trials
The company Curebase is promoting the concept of ‘bring-your-own-physician’ clinical trials.
Curebase – 2021
Taking the extra steps to ensure medical device quality assurance
Here’s how medical device manufacturers can go the extra mile to address regulatory challenges and user needs with comprehensive and purposeful quality assurance systems.
Eitan Medical – 2021
How to go beyond the regulatory requirements checklist
Many manufacturers run into issues as they adjust their devices and testing plans to meet updated regulatory requirements. Using a risk-based approach to predict and mitigate risks can help prevent costly setbacks.
WuXi AppTec – 2020
Here’s how you embrace regulatory controls to map medtech innovation
Using a properly formatted “roadmap” can help a medical device development team realize that the regulations are structured to yield innovation rather than stunt it.
MIDI Medical Product Development – 2020
Navigating regulations for in vitro diagnostics
Working through the regulatory requirements for IVD devices in your target markets can mean a successful launch into an industry segment poised for great growth in the coming years.
Intertek – 2020
Look outside your company for IP opportunities during unprecedented times
COVID-19 has caused many companies to hunker down, protect the status quo and embrace a conservative mindset. But some are taking this time to seek out intellectual property (IP) opportunities.
McAndrews, Held & Malloy – 2020
5 keys to stronger medtech agreements
It’s important to avoid missteps while protecting your medtech company’s innovations. Top Greenberg Traurig attorneys explain how.
Greenberg Traurig – 2020
How to create a strategic business plan to ensure success under MDR/IVDR
For many devices, the cost of compliance with MDR and IVDR will be high and will require significant expertise. Manufacturers should immediately begin developing a strategic business model that will prepare them for success under the new reforms.
Icon – 2019
Top 4 mistakes when implementing ISO 13485
ISO 13485 provides quality management system (QMS) guidelines for the production of safe and effective medical devices, but it’s easy to get tripped up.
Greenlight Guru – 2019
Medtech product liability and IP: Here’s where it’s going
Medtech is rapidly changing. Here’s how Greenberg Traurig experts think the shift will affect liability and IP protection.
Greenberg Traurig – 2019
Ready or not: How medical device manufacturers can prepare for E.U. MDR
The deadline to comply with new medical device regulations in the European Union is coming up fast. Here are some ideas on getting ready for the changes.
Sparta Systems – 2018
6 tips for quicker and stronger medtech patents
By developing a strategic patent portfolio quickly and successfully, medtech companies can navigate a path to commercial success.
Greenberg Traurig – 2018
5 tips for post-market medical device compliance
Post-market surveillance is an important process for medical device developers, with regulatory bodies expecting you to follow through and have effective procedures.
Greenlight Guru – 2018
Your device design looks great: Now how do you get reimbursement?
Until recently, the main focuses of medical device companies were designing devices and earning regulatory clearance for their new technologies. How times have changed. While innovative design and FDA approval are important for market entry, reimbursement is now a top concern for medical device CEOs, their investors and other stakeholders
RCRI – 2018
Medtech contracts versus purchase orders: Why contracts might be the better option
Contracts are underused by medical device companies. Here’s why they are worth a second look.
Medmarc Insurance Group – 2018
Here’s what you need to know about the new ISO 13485:2016
If your business manufactures, installs or services medical devices, you know it’s essential to transition to the new 13485: 2016 ISO standards as soon as possible.
EtQ – 2018
5 mistakes that can turn your product registration into a money drain
Medical device registration may seem straightforward but is actually prone to potentially costly mistakes.
Sparta Systems – 2017
How to protect your digital and mobile health innovations
Patent protection is becoming increasingly important for mobile health developers as more devices and applications join the connected world.
Greenberg Traurig – 2017
How to work with the new FDA
Here’s how medical device makers can capitalize on the strategic priorities and transparency initiatives at FDA’s CDRH.
RCRI – 2017
Intellectual property: How medtech startups can protect it
All companies begin with an idea. The details of protecting intellectual property, however, can be daunting, especially if your idea is in the field of medical technology.
Hamilton, Brook, Smith & Reynolds PC – 2017
Navigating the FDA for connected devices
As connectivity features become increasingly prevalent, developers are tasked with accounting for regulatory implications in increasingly long-term plans.
Ximedica – 2017
What you need to know about Europe’s new medical device rules
New regulations are coming to the European Union, and it’s up to developers to implement changes that will help them thrive in the new environment.
Icon – 2017
How to design a successful embolic protection devices clinical trial
Embolic protection devices could reduce risks of interventional cardiac procedures such as TAVR, but their efficacy is hard to prove.
Novella Clinical – 2017
How to work with a preclinical contract research organization
When outsourcing medical device laboratory testing, knowing what the regulatory and business requirements are up front is critical to the success of your testing program.
Toxikon– 2017
Medical device cybersecurity: Here’s how you verify and validate it
Cybersecurity is becoming an essential pillar of medical device design, and it’s important to find the right strategies to validate and verify your products are secure.
Intertek – 2017
Simplifying product testing and validation with fastener torque auditing
Capturing data during a product’s manufacturing process can help ensure the quality and consistency of that product. Here’s how that process plays out with fastener torque.
Futek – 2017
Validation and testing: What you need to know
In an increasingly competitive medical device industry, it’s still important that validation and testing is done right. Here are four things to keep in mind.
Jordi Labs – 2017







