Manufacturing, Machining and Molding
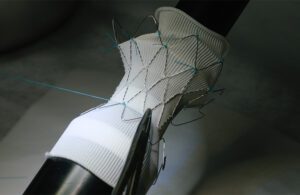
Each Medtronic Harmony valve is sown by hand with 2,200 to 2,500 stitches. [Photo courtesy of Medtronic]
How Medtronic manufactures its Harmony transcatheter pulmonary valve
Each Medtronic Harmony valve is sewn by hand to attach laser-cut pig tissue to the nitinol that makes this minimally invasive heart implant possible.
Medical Design & Outsourcing: Medtronic — 2023
Medical nitinol manufacturing: How this nickel-titanium alloy is made for medical devices
Nitinol (NiTi) might be the hottest material in the medical device industry as manufacturers find new applications for this superelastic, shape-memory, biocompatible metal.
Medical Design & Outsourcing — 2023
How 3D printing and surgical robotics enable Stryker’s cementless knee implants
With more than 1 million cementless knees implanted over a decade, Stryker Knee GM Lisa Kloes discusses tech that enables ortho innovation.
Medical Design & Outsourcing: Stryker — 2023

Automation in the form of collaborative robots, or “cobots,” can free employees up for more rewarding work. [Photo via iStock.com/Nay]
Collaborative robotics and Spanish-speaking shifts are helping Boston Scientific deal with a global labor shortage.
DeviceTalks: Boston Scientific – 2022
Leveraging automation to get ahead of supply and demand
Advances in robotics are helping manufacturers improve quality, fill labor shortages and promote personnel.
Donatelle – 2022
5 questions to ask when transitioning from medtech product development to manufacturing
Choosing a contract design or manufacturing partner begins a long, two-way relationship.
HS Design/SteriPack – 2022
Best practices when outsourcing medical injection molding
Part design, material selection, tooling and quality assurance are the keys to success.
Fictiv – 2022

Syringe filters contain thin membranes that, if damaged during assembly, would render them useless. [Photo courtesy of Emerson]
Emerson officials think their PulseStaking offering provides a new option for welding the small, delicate structures found in filtration parts.
Branson Welding and Assembly, Medical at Emerson – 2022
Three considerations for reshoring medical device components and assemblies
Moving production back to the United States requires strategic and complex navigation.
Beacon MedTech Solutions – 2022
Affordable 3D-printed medical devices reach commercialization
3D printer accessibility and affordability are enabling small device firms to develop and market a wave of personalized devices
Formlabs – 2022
How Stryker is using 3D printing to advance orthopedics
Orthopedic device giant Stryker uses additive manufacturing to make porous geometries that wouldn’t otherwise be possible.
DeviceTalks/Stryker – 2021
Salt baths increase stability in the medical device tempering process
Calibration baths, like salt baths, were originally for calibrating temperature assets. But there’s another use for them: manufacturing Nitinol medical devices.
Fluke Calibration – 2021
How additive manufacturing can complement MIM prototyping
When designing medical device components, you don’t need to choose between additive manufacturing and metal injection molding. Think of them as complementary.
Advanced Powder Products – 2021
Ramping up manufacturing for single-use surgical instruments: What you need to know
Manufacturers can optimize functionality, use and volume to deliver high-performing, high-quality single-use surgical instruments to customers.
Micro – 2021
How to evaluate laser performance for medical device manufacturing
Laser processing provides automation and efficiencies that help meet FDA regulations and produce superior medical device components.
ACSYS Lasertechnik US – 2021
Connected workers are key to meeting medical device manufacturing demands
Here’s how medical device manufacturers can navigate the “year of the connected worker.”
Delmiaworks – 2021
How to move medtech manufacturing into the future
“Factory of the future” systems can enable expanded and more versatile automation solutions, allowing device manufacturers to build their process steps, speed and cycles around the system’s capabilities.
Bosch Rexroth – 2020
3 things you need to scale up when outsourcing COVID-19 test production
Testing is one of the key efforts to control COVID-19, and U.S. diagnostic test production capabilities are in demand. To respond to the pandemic with urgency, test developers need a highly flexible, scalable supply chain that can move quickly, control costs and manage quality.
Web Industries – 2020
How to deliver high-volume, high-performing components with MIM
Medical device manufacturers can produce high-performing, complex geometric parts and components for surgical instruments at high volumes, cost-effectively using a metal injection molding (MIM) process.
Micro – 2020
When does in-house prototyping make sense?
Medical device companies have increasingly outsourced prototyping over the past two decades. It doesn’t have to be that way.
Kaleidoscope – 2020
Passing the test with vascular stents
Pulsatile durability testing is a time-consuming part of the vascular stent approval process. Accelerated test designs can deliver a high throughput when every specimen counts.
Instron – 2020
Diagnostic imaging plus additive manufacturing yields custom implants
Combining diagnostic imaging technologies with the design freedom of additive manufacturing has opened up new opportunities in prosthetics, enabling custom patient devices and improving the effectiveness of diagnosis, planning, surgery and clinical outcomes.
MT Ortho/GE Additive – 2020
Why it matters to have a skilled injection molding partner
A holistic approach enables a molder to guide medical device manufacturers from prototype to production.
Trelleborg – 2020
Metal injection molding can simplify the manufacture of complex parts
The MIM process combines the design flexibility of plastic injection molding with the strength and integrity of wrought metals.
Indo-MIM – 2020
How LaserSwiss can solve medtech manufacturers’ engineering challenges
Medtech companies can improve output quality and repeatability using the single-pass operation offered by LaserSwiss manufacturing.
OKAY Industries – 2019
Precision stamping for disposable medical components
Careful planning enables cost-effective production of high-quality products.
Metallon – 2019
How EU MDR will affect labeling on small medical devices
The new European Medical Device Regulation (EU MDR) Direct Part Marking (DPM) requirements challenge manufacturers of small-part and orthopedic devices to use the entirety of a part’s complex surface geometry for labeling.
Mack Molding – 2019
4 steps to solving tough automation challenges
As more dynamic automation systems take a larger role in the development of medical products, engineers need to learn how to design for repeatability and reliability.
Plexus – 2019
Here’s the case for single-source medical device manufacturing
Companies that outsource medical device manufacturing can either parse the production work among several providers or unify the effort from a single source. Both tactics have advantages, but the use of multiple suppliers can have expensive downsides.
B. Braun Medical – 2019
Making wearables and microfluidics manufacturable: What you need to know
Here’s why medical device creators need to design wearables and microfluidics manufacturability and scalability to achieve the optimal performance patients and providers rely on.
3M Medical Materials and Technologies – 2019
3 tips to avoid common medtech packaging blunders
A medical device packaging expert reveals three common packaging mistakes and how to avoid them.
Viant – 2019
Thermoelectric cooler solutions for medical applications
Active cooling solutions keep medical equipment below their maximum operating temperature to ensure proper performance.
Laird Thermal Systems – 2019
How going paperless can improve manufacturing speed and accuracy
A manufacturer that eliminates paper from its shop floor can save time and money while reducing errors.
Newcastle Systems – 2019
How to get your medical device to market faster
Developing a device from pre-clinical work through clinical trials and the regulatory process can take years and millions of dollars. Several factors can help a company to maintain a steady pace without breaking the bottom line.
RBC Medical Innovations – 2019
These common thermoplastics are ideal for medical device injection molding
The properties of certain polymers make them good choices for a variety of medical devices.
BMP Medical – 2019
Knowing the requirements of everyone involved in developing a rubber medical part during the design phase can speed the process right through production.
Apple Rubber Products – 2019
High consistency rubber versus liquid silicone rubber for medical device components
Here’s how high consistency rubber and liquid silicone rubber compare when it comes to making medical device components, according to ProMed (Plymouth, Minn.), which primarily works with the two types of silicone.
What is reel-to-reel molding?
A reel-to-reel method for electronics manufacturing eliminates waste and error for reduced costs.
Weiss-Aug Co. – 2019
Binder jetting and debinding: Here’s what you need to know
Binder jetting is a fast, affordable method of metal 3D printing. The right debinding fluids can harmlessly remove wax binders from metal 3D-printed parts before they’re sintered.
MicroCare Medical – 2019
What is an FDM printer and how does it work?
An FDM (fused deposition modeling) printer is a 3D printer that is often used in early concept development and prototyping for medical device design.
Kaleidoscope Innovation – 2019
What is a specification developer?
A specification developer can concentrate on developing a product idea, obtaining intellectual property protection and creating a sales plan — and leave the manufacturing to others.
Keystone Solutions Group – 2019
3 ways Carbon might have an edge with its 3D printing tech
Think of Carbon’s 3D printing technology enabling a host of customized medical
devices and parts.
Carbon – 2018
6 questions to ask when designing polymer components for medical devices
Choosing the right polymer for the job can be a daunting task. Here are the right questions to ask before you begin the process.
PolyOne Distribution – 2018
How to design liquid silicone rubber prototypes and components
Liquid silicone rubber (LSR) parts are used in a wide variety of medical devices. The process of molding LSR shares many similarities with conventional injection molding, but there are a few notable differences.
Protolabs – 2018
What you need to know about medical device packaging
Medical device packaging is certainly not easy. Here are some pitfalls you should avoid.
Keystone Solutions Group and Packaging Compliance Labs – 2018
How automated laser welding outperforms conventional welding for ring electrode manufacturing
While manual resistance welding is common in the device manufacturing industry,
automated electrode welding processes can enhance the consistency, quality, performance and efficiency of electrode ring assemblies.
Integer – 2018
How to simplify your supply chain while still mitigating risk
So many factors can disrupt a medtech manufacturer’s supply chain, sometimes with disastrous effects: geography, weather, management and quality control issues, just to name a few. Thoughtful planning about facility siting can make all the difference.
Tegra Medical – 2018
How to improve project management in a resource-constrained environment
Startup companies would be wise to heed the words of Andrew Carnegie: “Put all your eggs in one basket and watch that basket.” The “basket” is the project that will make or break a fledgling company, and the project manager’s job is to keep an eye on it.
Heraeus – 2018
Medical device packaging: It’s not a last-minute decision
Your medical device packaging design and related equipment are validated. Can you live with a hurried decision for the next 10 years?
Atlas Vac Machine – 2018
Crosshead construction for medical tubing: What you need to know
As the medical industry migrates more to multi-layer, multi-lumen tubing for various uses, the extrusion die design and manufacturing supply chain has likewise evolved. That includes new crosshead designs.
Guill Tool & Engineering – 2018
How six-axis robots are transforming medtech manufacturing
As industry 4.0 evolves, medical manufacturers are finding six-axis robots valuable all along the production line.
Stäubli Corp. – 2018
How medical equipment manufacturers tool up
Medical product tooling decisions play a major role in per part cost and turnaround time.
DureX – 2018
Why high-speed machining demands a high-end toolholder
In high-speed machining for medical equipment manufacturing, choosing the right toolholder is the key to greater precision, longer tool life and decreased machining costs.
Collis Toolholder – 2017
High-volume manufacturing: 4 points to consider before you scale up
Scaling to high-volume manufacturing requires companies to think ahead and prepare for the future early in the product lifecycle. Here are four points to reflect upon before your company scales up.
B. Braun Medical, OEM Division – 2017
How to select a micro-MIM supplier
With the development of increasingly smaller medical devices comes the challenge of identifying the best supplier and manufacturing method to meet extremely tight tolerances.
Donatelle – 2017
Why a global footprint is a medtech business imperative, not a buzzword
A global manufacturing footprint can help medical device companies capture value for their businesses by accessing talent and reducing costs. Here’s how to successfully execute this strategy without falling into a reactionary offshore initiative.
Preh IMA Automation – 2017
5 things you need to know about rapid injection molding
Here are five things you should consider when deciding to use rapid injection molding as part of your development process.
Vaupell Rapid Solutions – 2017
Dip molding medical device products: What you need to know
When it comes to dip molding products with emulsions of liquid rubber, it is necessary to complete a series of process steps to assure proper formation, vulcanization and finish treatment to meet the customer’s needs in the final application.
Kent Elastomer Products – 2017
Expanding design horizons with gas-assist molding
Gas-assist molding, the process of using nitrogen gas pressure to fully form a part, increases design and manufacturing options for injection molded components.
Mack Molding – 2017
Overmolding silicone onto thermoplastics: what you need for success
Overmolding silicone onto thermoplastics can be challenging, but offers advantages over TPEs that include chemical resistance, tensile strength and compression set. Here are some best practices to maximize your success.
MedPlast – 2017
What goes into injection molding cooling time?
Part design, material selection, mold design and processing all play a role when it comes to injection molding cooling time.
RJG – 2017
What is micromolding?
Micromolding is a very specialized art form. It is the tiny-scale molding form of injection molding that entails building a cavity to match the shape of the part you want to make, sort of like the plastic molding that makes Lego bricks.
Accumold – 2017
3D printing options are growing for medtech development: Here’s how
The new Multi Jet Fusion process out of HP is but the latest 3D printing technology to up the game for medical device development. Here’s a roundup of what’s available.
Protolabs – 2017
How have medtech launches and prototyping evolved with 3D printing?
Here are three basic ways that 3D printing can help you get your medical device project off the ground.
Keystone Solutions Group – 2017







