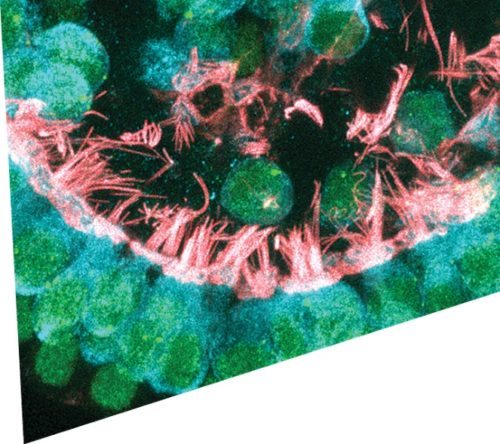
Newly formed cochlear hair cells contain intricate hair bundles with many stereocilia (critical for sensing sound) and other components that are critical for proper function and neural transmission. [Image courtesy of Will McLean/Frequency Therapeutics]
Frequency Therapeutics
Technology: Progenitor cell activation to treat hearing loss
CEO: David Lucchino
Website: www.frequencytx.com
Frequency Therapeutics is developing a pipeline of new drugs that activate progenitor cells within the body. The therapy has promise for a range of therapeutic indications for skin disorders, muscle regeneration and gastrointestinal diseases. Its lead product candidate stimulates regrowth of sensory hair cells in the inner ear to treat chronic noise-induced hearing loss.
Chronic noise-induced hearing loss is a significant disease effecting 48 million people in the United States and roughly 1.1 billion globally.
Frequency’s progenitor cell activation (PCA) is a precise and controlled approach that transiently causes Lgr5+ progenitor cells to divide and differentiate, as with naturally regenerating tissues such as the skin and intestine. It essentially mimics “stemness” in the body, potentially yielding an entirely new category of disease-modifying therapeutics for a wide range of degenerative conditions. The small molecules could have similar effects as gene therapy and CRISPR, with easier delivery. The hearing loss indication is moving to clinical trials within the next 12 to 18 months.
Company officials believe there is a broad potential for the PCA platform and announced the closing of a $32 million Series A financing in April to help expand its research.





Heather, great insights on the changing needs for medtech start-ups. We were thrilled to see, Madorra, one of our collaborating partners make your top 10. Look forward to following your updates on this incredible startup space.
They are doing some important work. Thanks, Juan. I’m excited to see the latest crop of startups for 2018 as well.