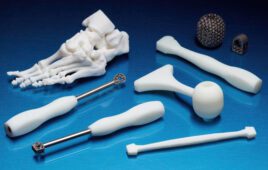
The 3D printers themselves get a lot of attention, but for 3D-printing to become ubiquitous in the medtech space, software will have to play a key role.
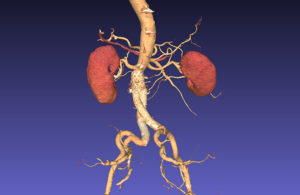
With “a few mouse clicks,” a radiologist on GE Healthcare’s Advantage Workstation can now separate volume renderings into both organs (kidneys) and blood — exporting them into 3D-printable files. (Note: The files are not cleared yet for diagnostic use.) [Image courtesy of GE Healthcare]
Still, there is a need for even more software advances if 3D printing is to drive widespread healthcare innovation — from surgeons training on patient-specific 3D-printed models to customized, printed orthopedic implants and other medical devices.
“That’s why I came to GE Healthcare — to help connect the dots,” Rader recently told Medical Design & Outsourcing.
Whether it involves spitting out a 3D-printable file off of a medical image, designing a more complicated 3D structure faster or ensuring that a 3D printer is truly printing to spec, there have been great strides in recent years in the software that powers the use of additive manufacturing in medtech.
“It’s inseparable because it is digital manufacturing. You can’t separate the computer out of the digital manufacturing process,” said Dr. Jenny Chen, founder and CEO of 3DHeals, a community of healthcare 3D-printing innovators.
Here are a few of the highlights of those software advances.
Quickly creating a 3D-printable file from medical images

It’s possible to take 3D-printable files from the GE Healthcare Advantage Workstation to create 3D prints from a range of materials — from color encoded to clear to preserve “X-ray vision.” [Image courtesy of GE Healthcare]
But Rader at GE Healthcare — replaying to MDO a demonstration he made at last year’s Radiological Society of North America convention — showed how a radiologist using the company’s Advantage Workstation within seconds can select 3D models of the right kidney and a particular branch of a blood vessel.
Physicians use Advantage Workstation to “read” patient scans like CT and MRI; once the radiologist defines the anatomy of interest, they can within a couple of mouse clicks see it exported to a 3D-printable file. It could be one of the STL files that 3D printers commonly use, or an alternative file format such as OBJ or 3MF or even VRML for virtual reality systems.
It’s been possible for years to create 3D-printable files from the Digital Imaging and Communications in Medicine (DICOM) output from CT or MRI scans. But the process involves interpreting the data on slices through the body, then segmenting organs, bones and vessels by outlining those structures in the slices. Manual segmentation processes are laborious, especially for a hospital radiologist trying to create the files amid a busy workday, according to Rader.
Advantage Workstation has helped radiology departments streamline the workflow for diagnostic reports For more than 20 years. What is new is the ability to re-use the 3D diagnostic visualizations for 3D printing and beyond via a simple file export.
“If you know manual segmentation and 3D printer interface software, you can do this today, but my hospital customers don’t often have a 3D-printing or engineering background,” Rader said. With the new GE Healthcare capability 3D Suite, he added, “You can simply, within three clicks, reuse the data you already generated to write the radiological report.”
GE for now is launching the new capability for creating 3D-printed educational models to aid in physician team communication and explain conditions to people seeking care, with plans to seek FDA permission for diagnostic and training uses.
“We have the ability to communicate in 3D,” Rader said.
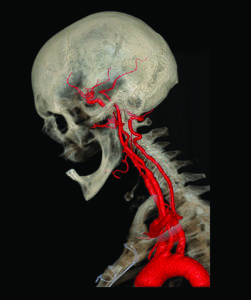
3D Reconstructed neurovasculature using Philips’ IntelliSpace Portal [Image courtesy of Philips]
When Philips launched the 10th version of its IntelliSpace Portal in 2017, it included an embedded 3D-modeling application meant to make it easier to generate and export 3D models for VR and printing as an extension of the clinical workflow.
Philips already had algorithms that could, for example, create a 3D version of a colon for a virtual colonoscopy or 3D versions of the heart or lungs, so it was fairly natural to make the jump to creating models that could actually be printed, said Kevin Lev, marketing director of advanced visualization and AI solutions at Philips.
“We have to remember that physical model creation is something radiologists are not necessarily used to doing on a day-to-day basis,” Lev said. “What we tried to do is make the process easy for the radiologist to do and transfer over to the surgical or intervention suite where there could be large benefits in surgical planning, potentially making a large difference for the patient.”
Also in 2017, Siemens Healthineers announced a partnership with Materialise — the first company to win FDA clearance for 3D printing anatomical models for diagnostic use — to incorporate Materialise Mimics inPrint software into Siemens’ advanced imaging platform Syngo.via.
“The easy workflows in the Materialise software make it easier for the radiologists and imaging technicians to prepare a file from the DICOM images to a 3D-printable file in a straightforward and easier way,” said Todd Pietila, who manages global business development for hospital 3D printing at Materialise.
Radiologists can use the Materialise software from any Syngo.via access point installed anywhere in a hospital network, according to Katrin Ganser, Syngo global marketing manager at Siemens Healthineers.
“We’re aiming to take the 3D printer to smaller hospitals that are not the traditional big research facilities,” Ganser said.
Faster and more intricate design

Anterior lumbar interbody fusion (ALIF) spinal implant with digitally-applied surface roughness and gradient periodic gyroid structure, made using nTop Platform software from nTopology [Image courtesy of nTopology]
For example, nTopology (New York) has design software that goes beyond traditional CAD by using mathematical equations to represent complex geometries, versus having to represent each complex feature separately. The result is faster design and smaller file sizes that are only megabytes in size, versus hundreds of megabytes or even gigabytes, according to Christopher Cho, senior application engineer at the company.
The 5-year-old company’s roots are in the engineering design industry. But nTopology has made inroads in medtech, especially in the orthopedic space where complex geometries inside implants have the potential to stimulate replacement bone growth.
Irish Manufacturing Research (IMR), for example, collaborated with nToplogy and British engineering company Renishaw and its Renishaw RenAM 500M metal AM system to produce lightweight spinal implants that mimic the mechanical properties of bone. It’s easier to make changes and experiment with the software because the alterations can then flow through the overall equations behind the design, according to Cho.
“They were able to use the software to explore more options that they could consider and test in a shorter amount of time,” he said of Renishaw and IMR. “We can give you a brand new, unique-looking structure, go to market with something that no one has ever seen before.”
Additive manufacturing excels at creating complex technologies, so nTopology’s design software marries well with 3D printing. The company has partnerships with Renishaw, EOS and others to integrate nToplogy directly into their 3D-printing systems, versus relying on STL files and their limitations.
“If we can avoid that digital step, work directly with the machine manufacturers … basically integrate the digital model workflow directly with them, we would be able to bypass this digital obstacle and really push the envelope on the design into the manufacturing process,” Cho said.
The rise of in situ monitoring

3D Systems’ DMP Flex 350 machine is equipped with the latest monitoring software for maintenance tracking, as well as in-process monitoring of the build quality. [Image courtesy of 3D Systems]
3D Systems, for example, has in-process monitoring systems in metal 3D printing systems, including its DMP Flex 350, DMP Factory 350 and DMP Factory 500, with data analysis taking place post-process.
Plans include releasing new software for automated monitoring analysis soon to aid users in assessing part quality, said Markus Reichmann, healthcare business development manager at the printer company. “We are really putting a lot of effort into automated monitoring.”
3D Systems uses a digital camera and a light diode that collects emitted light to tell exactly what is going on in the melt pool.
“A next step will be to incorporate the online analysis that would run during the printing … and provide a feedback loop to the printing process based on the analysis results,” Reichmann said.

For in-situ process monitoring, the Origin One boasts two optical cameras, three infrared cameras, humidity sensors, temperature sensors and more — as well as analytical software to better figure out what is going on. [Image courtesy of Origin]
The reason for so many controls is that Origin grew out of partnerships with major materials companies such as BASF and Henkel, which are also important medical device industry suppliers. They wanted a printer they could fine-tune to build the materials that designers in medtech and other advanced industries want to use.
Henkel, for example, has silicones that are used in medical devices, although silicones are notoriously hard to 3D print.
“Not many [3D-printer] companies out there offer it because it’s hard to do fine features, but through our software controls, through the kind of tweaks, we’re able to get the silicones working on our system when they’ve failed with every other resin-based 3D printer out there,” Watterson said.
Using Origin’s print process, silicones can be printed within a 100-micron tolerance, and with rigid materials, a 50-micron or lower tolerance can be achieved.
Origin, Watterson said, is big on data access and analysis. “We want to make everything open and accessible, create really good hardware and not try to do everything. … We want to broaden and work with companies to co-develop applications.”
In situ monitoring and data access through software are crucial for medical 3D printing innovation, Chen said. “It’s extremely important to remove the human element so that it’s no longer an art, so it’s not like one product can be studied differently from the other, but consistently deliver with quality.”